Graphene-based dental adhesive with anti-biofilm activity
- PMID: 29233187
- PMCID: PMC5728064
- DOI: 10.1186/s12951-017-0322-1
Graphene-based dental adhesive with anti-biofilm activity
Abstract
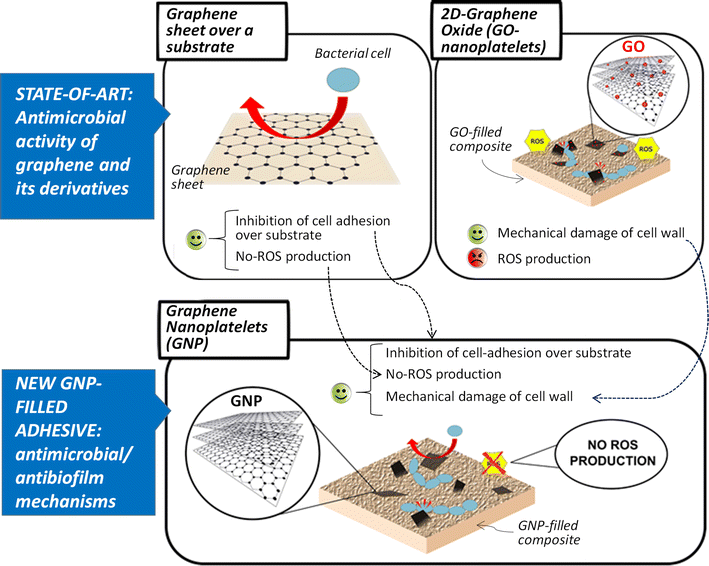
Background: Secondary caries are considered the main cause of dental restoration failure. In this context, anti-biofilm and bactericidal properties are desired in dental materials against pathogens such as Streptococcus mutans. To this purpose, graphene based materials can be used as fillers of polymer dental adhesives. In this work, we investigated the possibility to use as filler of dental adhesives, graphene nanoplatelets (GNP), a non toxic hydrophobic nanomaterial with antimicrobial and anti-biofilm properties.
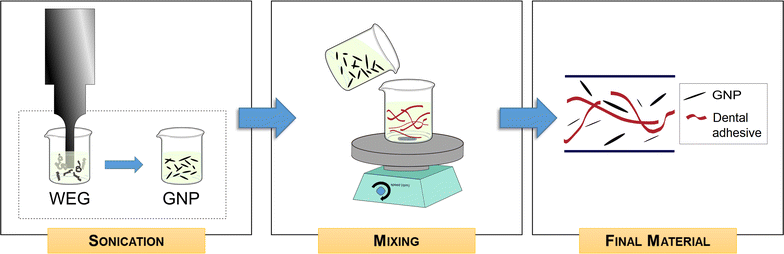
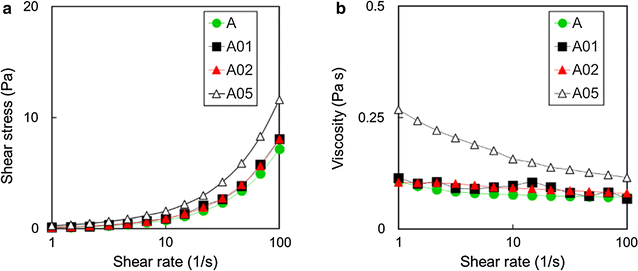
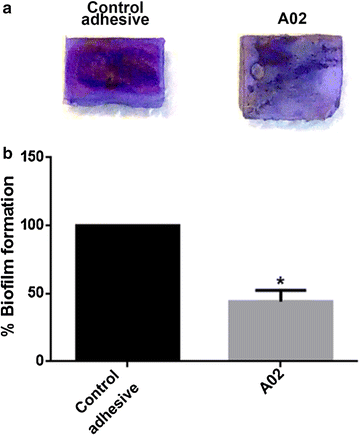
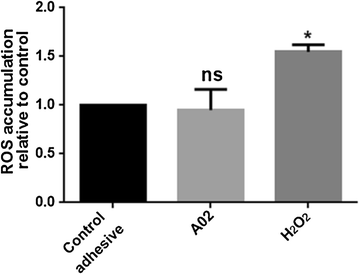
Results: Graphene nanoplatelets have been produced starting from graphite intercalated compounds through a process consisting of thermal expansion and liquid exfoliation. Then, a dental adhesive filled with GNPs at different volume fractions has been produced through a solvent evaporation method. The rheological properties of the new experimental adhesives have been assessed experimentally. The adhesive properties have been tested using microtensile bond strength measurements (µ-TBS). Biocidal activity has been studied using the colony forming units count (CFU) method. The anti-biofilm properties have been demonstrated through FE-SEM imaging of the biofilm development after 3 and 24 h of growth.
Conclusions: A significantly lower vitality of S. mutans cells has been demonstrated when in contact with the GNP filled dental adhesives. Biofilm growth on adhesive-covered dentine tissues demonstrated anti-adhesion properties of the produced materials. µ-TBS results demonstrated no significant difference in µ-TBS between the experimental and the control adhesive. The rheology tests highlighted the necessity to avoid low shear rate regimes during adhesive processing and application in clinical protocol, and confirmed that the adhesive containing the 0.2%wt of GNPs possess mechanical properties comparable with the ones of the control adhesive.
Figures





References
-
- De Almeida Neves A, Coutinho E, Cardoso MV, Lambrechts P, Van Meerbeek B. Current concepts and techniques for caries excavation and adhesion to residual dentin. J Adhes Dent. 2011;13:7–22. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

