The RIO protein kinase-encoding gene Sj-riok-2 is involved in key reproductive processes in Schistosoma japonicum
- PMID: 29233188
- PMCID: PMC5727939
- DOI: 10.1186/s13071-017-2524-7
The RIO protein kinase-encoding gene Sj-riok-2 is involved in key reproductive processes in Schistosoma japonicum
Abstract
Background: Schistosomiasis is one of the most prevalent parasitic diseases worldwide and is caused by parasitic trematodes of the genus Schistosoma. The pathogenesis of schistosomiasis is caused by eggs whose production is the consequence of the pairing of schistosomes and the subsequent sexual maturation of the female. Previous studies have demonstrated that protein kinases are involved in processes leading to the male-induced differentiation of the female gonads, ovary and vitellarium. Right open reading frame protein kinase 2 (RIOK-2) is a member of the atypical kinase family and shown in other organisms to be responsible for ribosomal RNA biogenesis and cell-cycle progression, as well as involves in nematode development. However, nothing is known about its functions in any trematode including schistosome.
Methods: We isolated and characterized the riok-2 gene from S. japonicum, and detected the transcriptional profiles of Sj-riok-2 by using real-time PCR and in situ hybridization. RNAi-mediated knockdown of Sj-riok-2 was performed, mitotic activities were detected by EdU incorporation assay and morphological changes on organs were observed by confocal laser scanning microscope (CLSM).
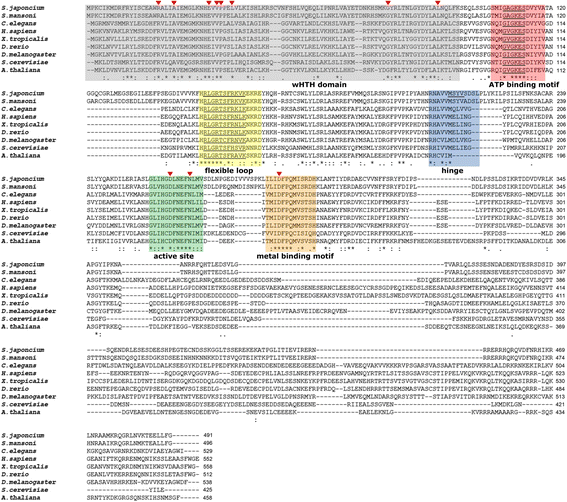
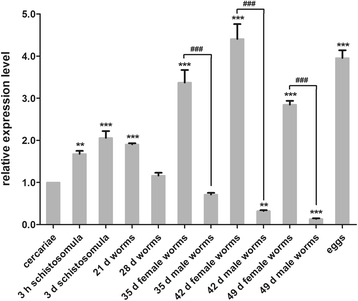
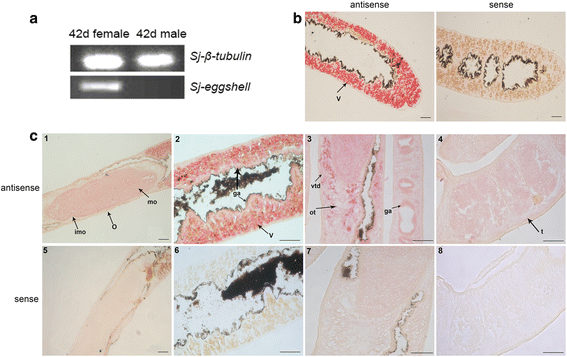
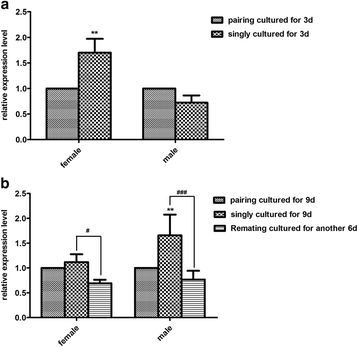
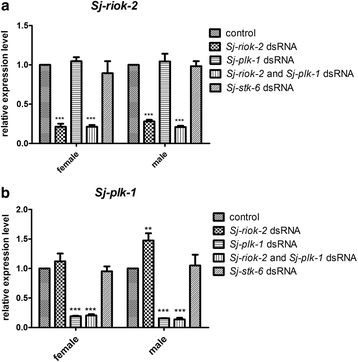
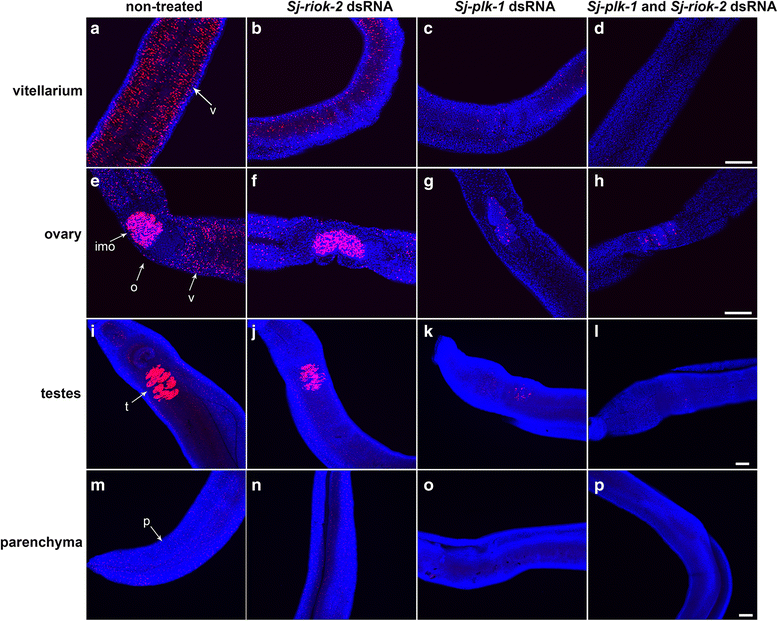
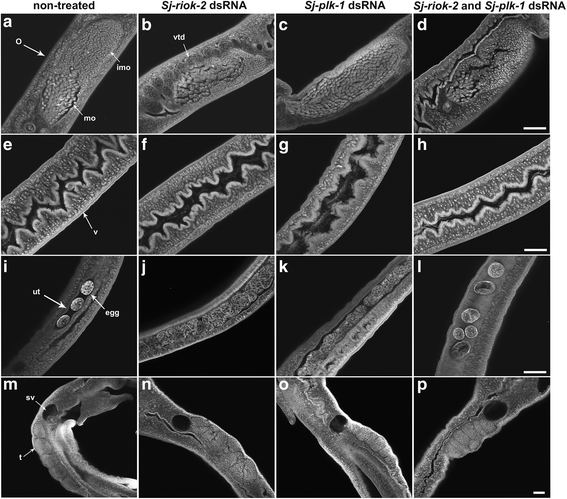
Results: In silico analyses of the amino acid sequence of Sj-RIOK-2 revealed typical features of this class of kinases including a winged helix (wHTH) domain and a RIO kinase domain. Sj-riok-2 is transcribed in different developmental stages of S. japonicum, with a higher abundance in adult females and eggs. Localization studies showed that Sj-riok-2 was mainly transcribed in female reproductive organs. Experiments with adult schistosomes in vitro demonstrated that the transcriptional level of Sj-riok-2 was affected by pairing. Knocking down Sj-riok-2 by RNAi reduced cell proliferation in the vitellarium and caused the increased amount of mature oocytes in ovary and an accumulation of eggs within the uterus.
Conclusions: Sj-riok-2 is involved in the reproductive development and maturation of female S. japonicum. Our findings provide first evidence for a pairing-dependent role of Sj-riok-2 in the reproductive development and maturation of female S. japonicum. Thus this study contributes to the understanding of molecular processes controlling reproduction in schistosomes.
Keywords: Gonad; RIO2 kinase; RNA interference; Reproductive development; Schistosoma japonicum.
Conflict of interest statement
Ethics approval and consent to participate
The care and maintenance of experimental mice in this study was approved by the Institutional Animal Care and Use Committee of Huazhong Agricultural University according to the Regulations for the Administration of Affairs Concerning Experimental Animals of Hubei Province, P.R. China.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
First Evidence of Function for Schistosoma japonicumriok-1 and RIOK-1.Pathogens. 2021 Jul 8;10(7):862. doi: 10.3390/pathogens10070862. Pathogens. 2021. PMID: 34358012 Free PMC article.
-
Differential gene expression, including Sjfs800, in Schistosoma japonicum females at pre-pairing, initial pairing and oviposition.Parasit Vectors. 2019 Aug 23;12(1):414. doi: 10.1186/s13071-019-3672-8. Parasit Vectors. 2019. PMID: 31443730 Free PMC article.
-
Exploring features and function of Ss-riok-3, an enigmatic kinase gene from Strongyloides stercoralis.Parasit Vectors. 2014 Dec 5;7:561. doi: 10.1186/s13071-014-0561-z. Parasit Vectors. 2014. PMID: 25477034 Free PMC article.
-
Schistosoma mansoni: signal transduction processes during the development of the reproductive organs.Parasitology. 2010 Mar;137(3):497-520. doi: 10.1017/S0031182010000053. Epub 2010 Feb 18. Parasitology. 2010. PMID: 20163751 Review.
-
Invasion by schistosome cercariae: neglected aspects in Schistosoma japonicum.Trends Parasitol. 2004 Sep;20(9):397-400. doi: 10.1016/j.pt.2004.06.006. Trends Parasitol. 2004. PMID: 15324727 Review.
Cited by
-
First Evidence of Function for Schistosoma japonicumriok-1 and RIOK-1.Pathogens. 2021 Jul 8;10(7):862. doi: 10.3390/pathogens10070862. Pathogens. 2021. PMID: 34358012 Free PMC article.
-
Establishment of an Animal Model Scheme of Strongyloides stercoralis-Infected Meriones meridianus.Pathogens. 2023 Oct 26;12(11):1285. doi: 10.3390/pathogens12111285. Pathogens. 2023. PMID: 38003750 Free PMC article.
-
Deep phosphoproteome analysis of Schistosoma mansoni leads development of a kinomic array that highlights sex-biased differences in adult worm protein phosphorylation.PLoS Negl Trop Dis. 2020 Mar 23;14(3):e0008115. doi: 10.1371/journal.pntd.0008115. eCollection 2020 Mar. PLoS Negl Trop Dis. 2020. PMID: 32203512 Free PMC article.
-
Docking-Based Virtual Screening Enables Prioritizing Protein Kinase Inhibitors With In Vitro Phenotypic Activity Against Schistosoma mansoni.Front Cell Infect Microbiol. 2022 Jul 5;12:913301. doi: 10.3389/fcimb.2022.913301. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35865824 Free PMC article.
-
Effects of programmed cell death protein 10 on fecundity in Schistosoma japonicum.Parasitol Res. 2020 Apr;119(4):1317-1325. doi: 10.1007/s00436-020-06635-1. Epub 2020 Mar 9. Parasitol Res. 2020. PMID: 32152713
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

