Generation of Adrenal Chromaffin-like Cells from Human Pluripotent Stem Cells
- PMID: 29233551
- PMCID: PMC5768882
- DOI: 10.1016/j.stemcr.2017.11.003
Generation of Adrenal Chromaffin-like Cells from Human Pluripotent Stem Cells
Abstract
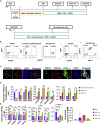
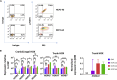
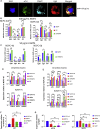
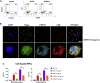
Adrenomedullary chromaffin cells are catecholamine (CA)-producing cells originating from trunk neural crest (NC) via sympathoadrenal progenitors (SAPs). We generated NC and SAPs from human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) in vitro via BMP2/FGF2 exposure, ascertained by qPCR and immunoexpression of SOX10, ASCL1, TFAP2α, and PHOX2B, and by fluorescence-activated cell sorting selection for p75NTR and GD2, and confirmed their trunk-like HOX gene expression. We showed that continuing BMP4 and curtailing FGF2 in vitro, augmented with corticosteroid mimetic, induced these cells to upregulate the chromaffin cell-specific marker PNMT and other CA synthesis and storage markers, and we demonstrated noradrenaline and adrenaline by Faglu and high-performance liquid chromatography. We showed these human cells' SAP-like property of migration and differentiation into cells expressing chromaffin cell markers by implanting them into avian embryos in vivo and in chorio-allantoic membrane grafts. These cells have the potential for investigating differentiation of human chromaffin cells and for modeling diseases involving this cell type.
Keywords: chromaffin cells; human embryonic stem cells; human induced pluripotent cells; neural crest; sympathoadrenal progenitors.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Allmendinger A., Stoeckel E., Saarma M., Unsicker K., Huber K. Development of adrenal chromaffin cells is largely normal in mice lacking the receptor tyrosine kinase c-Ret. Mech. Dev. 2003;120:299–304. - PubMed
-
- Anderson D.J. Molecular control of cell fate in the neural crest: the sympathoadrenal lineage. Annu. Rev. Neurosci. 1993;16:129–158. - PubMed
-
- Anderson D.J., Carnahan J.F., Michelsohn A., Patterson P.H. Antibody markers identify a common progenitor to sympathetic neurons and chromaffin cells in vivo and reveal the timing of commitment to neuronal differentiation in the sympathoadrenal lineage. J. Neurosci. 1991;11:3507–3519. - PMC - PubMed
-
- Attie-Bitach T., Abitbol M., Gerard M., Delezoide A.L., Auge J., Pelet A., Amiel J., Pachnis V., Munnich A., Lyonnet S. Expression of the RET proto-oncogene in human embryos. Am. J. Med. Genet. 1998;80:481–486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

