mTORC1 as the main gateway to autophagy
- PMID: 29233869
- PMCID: PMC5869864
- DOI: 10.1042/EBC20170027
mTORC1 as the main gateway to autophagy
Abstract
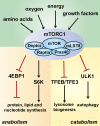
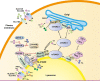
Cells and organisms must coordinate their metabolic activity with changes in their environment to ensure their growth only when conditions are favourable. In order to maintain cellular homoeostasis, a tight regulation between the synthesis and degradation of cellular components is essential. At the epicentre of the cellular nutrient sensing is the mechanistic target of rapamycin complex 1 (mTORC1) which connects environmental cues, including nutrient and growth factor availability as well as stress, to metabolic processes in order to preserve cellular homoeostasis. Under nutrient-rich conditions mTORC1 promotes cell growth by stimulating biosynthetic pathways, including synthesis of proteins, lipids and nucleotides, and by inhibiting cellular catabolism through repression of the autophagic pathway. Its close signalling interplay with the energy sensor AMP-activated protein kinase (AMPK) dictates whether the cell actively favours anabolic or catabolic processes. Underlining the role of mTORC1 in the coordination of cellular metabolism, its deregulation is linked to numerous human diseases ranging from metabolic disorders to many cancers. Although mTORC1 can be modulated by a number of different inputs, amino acids represent primordial cues that cannot be compensated for by any other stimuli. The understanding of how amino acids signal to mTORC1 has increased considerably in the last years; however this area of research remains a hot topic in biomedical sciences. The current ideas and models proposed to explain the interrelationship between amino acid sensing, mTORC1 signalling and autophagy is the subject of the present review.
Keywords: amino acids; autophagy; lysosome; mTOR.
© 2017 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

References
-
- Ravikumar B., Sarkar S., Davies J.E., Futter M., Garcia-Arencibia M., Green-Thompson Z.W. et al. (2010) Regulation of mammalian autophagy in physiology and pathophysiology. Physiol. Rev. 90, 1383–1435 - PubMed
-
- Guertin D.A. and Sabatini D.M. (2007) Defining the role of mTOR in cancer. Cancer Cell 12, 9–22 - PubMed
-
- Hara K., Maruki Y., Long X., Yoshino K.-i., Oshiro N., Hidayat S. et al. (2002) Raptor, a Binding Partner of Target of Rapamycin (TOR), Mediates TOR Action. Cell 110, 177–189 - PubMed
-
- Kim D.-H., Sarbassov D.D., Ali S.M., King J.E., Latek R.R., Erdjument-Bromage H. et al. (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175 - PubMed
-
- Jacinto E., Loewith R., Schmidt A., Lin S., Ruegg M.A., Hall A. et al. (2004) Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 6, 1122–1128 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

