Structural insights into the mechanisms of CNBD channel function
- PMID: 29233886
- PMCID: PMC5806680
- DOI: 10.1085/jgp.201711898
Structural insights into the mechanisms of CNBD channel function
Abstract
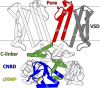
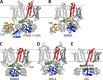
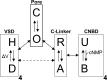
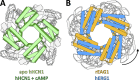
Cyclic nucleotide-binding domain (CNBD) channels are a family of ion channels in the voltage-gated K+ channel superfamily that play crucial roles in many physiological processes. CNBD channels are structurally similar but functionally very diverse. This family includes three subfamilies: (1) the cyclic nucleotide-gated (CNG) channels, which are cation-nonselective, voltage-independent, and cyclic nucleotide-gated; (2) the hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, which are weakly K+ selective, hyperpolarization-activated, and cyclic nucleotide-gated; and (3) the ether-à-go-go-type (KCNH) channels, which are strongly K+ selective, depolarization-activated, and cyclic nucleotide-independent. Recently, several high-resolution structures have been reported for intact CNBD channels, providing a structural framework to better understand their diverse function. In this review, we compare and contrast the recent structures and discuss how they inform our understanding of ion selectivity, voltage-dependent gating, and cyclic nucleotide-dependent gating within this channel family.
© 2018 James and Zagotta.
Figures







References
-
- Akimoto M., Zhang Z., Boulton S., Selvaratnam R., VanSchouwen B., Gloyd M., Accili E.A., Lange O.F., and Melacini G.. 2014. A mechanism for the auto-inhibition of hyperpolarization-activated cyclic nucleotide-gated (HCN) channel opening and its relief by cAMP. J. Biol. Chem. 289:22205–22220. 10.1074/jbc.M114.572164 - DOI - PMC - PubMed
-
- Altomare C., Terragni B., Brioschi C., Milanesi R., Pagliuca C., Viscomi C., Moroni A., Baruscotti M., and DiFrancesco D.. 2003. Heteromeric HCN1-HCN4 channels: a comparison with native pacemaker channels from the rabbit sinoatrial node. J. Physiol. 549:347–359. 10.1113/jphysiol.2002.027698 - DOI - PMC - PubMed
-
- Bankston J.R., Camp S.S., DiMaio F., Lewis A.S., Chetkovich D.M., and Zagotta W.N.. 2012. Structure and stoichiometry of an accessory subunit TRIP8b interaction with hyperpolarization-activated cyclic nucleotide-gated channels. Proc. Natl. Acad. Sci. USA. 109:7899–7904. 10.1073/pnas.1201997109 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

