Implication of 4E-BP1 protein dephosphorylation and accumulation in pancreatic cancer cell death induced by combined gemcitabine and TRAIL
- PMID: 29233971
- PMCID: PMC5870593
- DOI: 10.1038/s41419-017-0001-z
Implication of 4E-BP1 protein dephosphorylation and accumulation in pancreatic cancer cell death induced by combined gemcitabine and TRAIL
Abstract
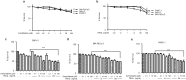
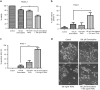
Pancreatic cancer cells show varying sensitivity to the anticancer effects of gemcitabine. However, as a chemotherapeutic agent, gemcitabine can cause intolerably high levels of toxicity and patients often develop resistance to the beneficial effects of this drug. Combination studies show that use of gemcitabine with the pro-apoptotic cytokine TRAIL can enhance the inhibition of survival and induction of apoptosis of pancreatic cancer cells. Additionally, following combination treatment there is a dramatic increase in the level of the hypophosphorylated form of the tumour suppressor protein 4E-BP1. This is associated with inhibition of mTOR activity, resulting from caspase-mediated cleavage of the Raptor and Rictor components of mTOR. Use of the pan-caspase inhibitor Z-VAD-FMK indicates that the increase in level of 4E-BP1 is also caspase-mediated. ShRNA-silencing of 4E-BP1 expression renders cells more resistant to cell death induced by the combination treatment. Since the levels of 4E-BP1 are relatively low in untreated pancreatic cancer cells these results suggest that combined therapy with gemcitabine and TRAIL could improve the responsiveness of tumours to treatment by elevating the expression of 4E-BP1.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

