Adeno-associated virus serotype rh10 is a useful gene transfer vector for sensory nerves that innervate bone in immunodeficient mice
- PMID: 29233995
- PMCID: PMC5727257
- DOI: 10.1038/s41598-017-17393-z
Adeno-associated virus serotype rh10 is a useful gene transfer vector for sensory nerves that innervate bone in immunodeficient mice
Abstract
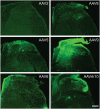
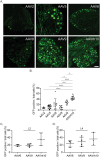
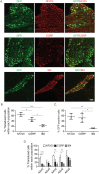
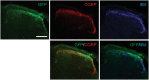
Adeno-associated virus (AAV) is frequently used to manipulate gene expression in the sensory nervous system for the study of pain mechanisms. Although some serotypes of AAV are known to have nerve tropism, whether AAV can distribute to sensory nerves that innervate the bone or skeletal tissue has not been shown. This information is crucial, since bone pain, including cancer-induced bone pain, is an area of high importance in pain biology. In this study, we found that AAVrh10 transduces neurons in the spinal cord and dorsal root ganglia of immunodeficient mice with higher efficacy than AAV2, 5, 6, 8, and 9 when injected intrathecally. Additionally, AAVrh10 has tropism towards sensory neurons in skeletal tissue, such as bone marrow and periosteum, while it occasionally reaches the sensory nerve fibers in the mouse footpad. Moreover, AAVrh10 has higher tropic affinity to large myelinated and small peptidergic sensory neurons that innervate bone, compared to small non-peptidergic sensory neurons that rarely innervate bone. Taken together, these results suggest that AAVrh10 is a useful gene delivery vector to target the sensory nerves innervating bone. This finding may lead to a greater understanding of the molecular mechanisms of chronic bone pain and cancer-induced bone pain.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

