The Differentiation Stage of Transplanted Stem Cells Modulates Nerve Regeneration
- PMID: 29234013
- PMCID: PMC5727226
- DOI: 10.1038/s41598-017-17043-4
The Differentiation Stage of Transplanted Stem Cells Modulates Nerve Regeneration
Abstract
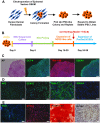
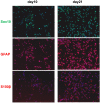
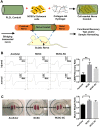
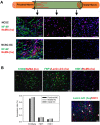
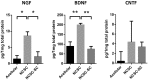
In regenerative medicine applications, the differentiation stage of implanted stem cells must be optimized to control cell fate and enhance therapeutic efficacy. We investigated the therapeutic potential of human induced pluripotent stem cell (iPSC)-derived cells at two differentiation stages on peripheral nerve regeneration. Neural crest stem cells (NCSCs) and Schwann cells (NCSC-SCs) derived from iPSCs were used to construct a tissue-engineered nerve conduit that was applied to bridge injured nerves in a rat sciatic nerve transection model. Upon nerve conduit implantation, the NCSC group showed significantly higher electrophysiological recovery at 1 month as well as better gastrocnemius muscle recovery at 5 months than the acellular group, but the NCSC-SC group didn't. Both transplanted NCSCs and NCSC-SCs interacted with newly-growing host axons, while NCSCs showed better survival rate and distribution. The transplanted NCSCs mainly differentiated into Schwann cells with no teratoma formation, and they secreted higher concentrations of brain-derived neurotrophic factor and nerve growth factor than NCSC-SCs. In conclusion, transplantation of iPSC-NCSCs accelerated functional nerve recovery with the involvement of stem cell differentiation and paracrine signaling. This study unravels the in vivo performance of stem cells during tissue regeneration, and provides a rationale of using appropriate stem cells for regenerative medicine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

