Progress in Nanomedicine: Approved and Investigational Nanodrugs
- PMID: 29234213
- PMCID: PMC5720487
Progress in Nanomedicine: Approved and Investigational Nanodrugs
Abstract
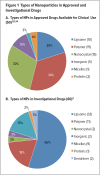
Nanomedicine is a relatively new and rapidly evolving field combining nanotechnology with the biomedical and pharmaceutical sciences.1-3 Nanoparticles (NPs) can impart many pharmacokinetic, efficacy, safety, and targeting benefits when they are included in drug formulations.1-5 Many nanodrugs have entered clinical practice, and even more are being investigated in clinical trials for a wide variety of indications.2 However, nanopharmaceuticals also face challenges, such as the need for better characterization, possible toxicity issues, a lack of specific regulatory guidelines, cost-benefit considerations, and waning enthusiasm among some health care professionals. 4,5 For these reasons, expectations regarding nanodrugs that are in early stages of development or clinical trials need to remain realistic.4.
Figures


References
-
- Bobo D, Robinson KJ, Islam J, et al. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33(10):2373–2387. - PubMed
-
- Caster JM, Patel AN, Zhang T, Wang A. Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(1) - PubMed
-
- Havel HA. Where are the nanodrugs? An industry perspective on development of drug products containing nanomaterials. AAPS J. 2016;18(6):1351–1353. - PubMed
-
- Havel H, Finch G, Strode P, et al. Nanomedicines: from bench to bedside and beyond. AAPS J. 2016;18(6):1373–1378. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
