MicroRNAs and metastasis: small RNAs play big roles
- PMID: 29234933
- PMCID: PMC5803344
- DOI: 10.1007/s10555-017-9712-y
MicroRNAs and metastasis: small RNAs play big roles
Abstract
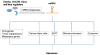
MicroRNAs (miRNAs) are small non-coding RNAs regulating post-transcriptional gene expression. They play important roles in many biological processes under physiological or pathological conditions, including development, metabolism, tumorigenesis, metastasis, and immune response. Over the past 15 years, significant insights have been gained into the roles of miRNAs in cancer. Depending on the cancer type, miRNAs can act as oncogenes, tumor suppressors, or metastasis regulators. In this review, we focus on the role of miRNAs as components of molecular networks regulating metastasis. These miRNAs, termed metastamiRs, promote or inhibit metastasis through various mechanisms, including regulation of migration, invasion, colonization, cancer stem cell properties, epithelial-mesenchymal transition, and microenvironment. Some of these metastamiRs represent attractive therapeutic targets for cancer treatment.
Keywords: MetastamiR; Metastasis; MicroRNA (miRNA); OncomiR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. - PubMed
-
- Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11(3):228–234. - PubMed
-
- Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, et al. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432(7014):235–240. - PubMed
-
- Han J, Lee Y, Yeom KH, Nam JW, Heo I, Rhee JK, et al. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell. 2006;125(5):887–901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

