Interferon α2 and interferon γ induce the degranulation independent production of VEGF-A and IL-1 receptor antagonist and other mediators from human mast cells
- PMID: 29235261
- PMCID: PMC5818443
- DOI: 10.1002/iid3.211
Interferon α2 and interferon γ induce the degranulation independent production of VEGF-A and IL-1 receptor antagonist and other mediators from human mast cells
Abstract
Background: Mast cells are resident immune effector cells, often studied in the context of allergic disease. Found in substantial numbers at sites of potential infection they are increased at sites of angiogenesis and can be pivotal for the sensing and clearance of a variety of pathogens. Interferons (IFNs) are cytokines that are critical for host defence against intracellular pathogens. Increased levels of IFNs are observed during viral infection and in autoimmune diseases. IFNs are also widely used therapeutically and have been examined in the therapy of severe asthma.
Objective: To define the selective human mast cell cytokine and chemokine response following activation with type I or type II IFN's.
Methods: The ability of both IFNα2 and IFNγ to induce cytokine production by human cord blood-derived mast cells was examined in vitro. Cytokine and chemokine production at 6 and 24 h was assessed by multiplex protein analysis. Degranulation was assessed by β-hexosaminidase release. Mast cells were also treated with reovirus or respiratory syncytial virus and their production of CXCL10, IL-1 receptor antagonist (IL-1Ra), and vascular endothelial growth factor (VEGF) examined after 24 h.
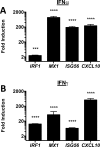
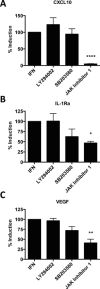
Results: In addition to increased expression of classical IFN response genes, such as CXCL10, small but significant increases in CCL5 and IL-17 production were observed following IFN activation. Notably, human mast cells produced both VEGF and IL-1Ra in a dose dependent manner. These responses occurred in the absence of mast cell degranulation by a mechanism consistent with classical IFN signaling. Both reovirus and respiratory syncytial virus infection of mast cells, were also associated with IFN-dependent IL-1Ra expression.
Conclusion and clinical relevance: Our findings demonstrate that IFNs have profound impact on cytokine and chemokine expression by human mast cells, alone or in the context of viral infection. Mast cell VEGF and IL-1Ra responses to IFNs could impact the regulation of local inflammatory responses and subsequent tissue remodeling.
Keywords: CXCL10; IL-1Ra; VEGF; interferons; mast cells; reovirus; respiratory syncytial virus.
© 2017 The Authors. Immunity, Inflammation and Disease Published by John Wiley & Sons Ltd.
Figures





References
-
- Oldford, S. A. , and Marshall J. S.. 2013. Mast cell modulation of the tumor microenvironment pp. 479–509. in Shurin M. R., Umansky V., and Maliguine A., eds. The tumor immunoenvironment. Springer Netherlands, Dordrecht.
-
- Norrby, K. 2002. Mast cells and angiogenesis. APMIS 110(5):355–371. PubMed PMID: 12076253. - PubMed
-
- Dawicki, W. , and Marshall J. S.. 2007. New and emerging roles for mast cells in host defence. Curr. Opin. Immunol. 19(1):31–38. PubMed PMID: 17126541. - PubMed
-
- Fischer, M. , Harvima I. T., Carvalho R. F., Moller C., Naukkarinen A., Enblad G., and Nilsson G.. 2006. Mast cell CD30 ligand is upregulated in cutaneous inflammation and mediates degranulation‐independent chemokine secretion. J. Clin. Invest. 116(10):2748–2756. PubMed PMID: 16964309. Pubmed Central PMCID: 1560346. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

