In vivo metabolic imaging of Traumatic Brain Injury
- PMID: 29235509
- PMCID: PMC5727520
- DOI: 10.1038/s41598-017-17758-4
In vivo metabolic imaging of Traumatic Brain Injury
Abstract
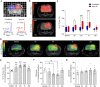
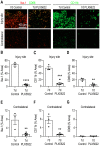
Complex alterations in cerebral energetic metabolism arise after traumatic brain injury (TBI). To date, methods allowing for metabolic evaluation are highly invasive, limiting our understanding of metabolic impairments associated with TBI pathogenesis. We investigated whether 13C MRSI of hyperpolarized (HP) [1-13C] pyruvate, a non-invasive metabolic imaging method, could detect metabolic changes in controlled cortical injury (CCI) mice (n = 57). Our results show that HP [1-13C] lactate-to-pyruvate ratios were increased in the injured cortex at acute (12/24 hours) and sub-acute (7 days) time points after injury, in line with decreased pyruvate dehydrogenase (PDH) activity, suggesting impairment of the oxidative phosphorylation pathway. We then used the colony-stimulating factor-1 receptor inhibitor PLX5622 to deplete brain resident microglia prior to and after CCI, in order to confirm that modulations of HP [1-13C] lactate-to-pyruvate ratios were linked to microglial activation. Despite CCI, the HP [1-13C] lactate-to-pyruvate ratio at the injury cortex of microglia-depleted animals at 7 days post-injury remained unchanged compared to contralateral hemisphere, and PDH activity was not affected. Altogether, our results demonstrate that HP [1-13C] pyruvate has great potential for in vivo non-invasive detection of cerebral metabolism post-TBI, providing a new tool to monitor the effect of therapies targeting microglia/macrophages activation after TBI.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

