Biodegradable Bisvinyl Sulfonemethyl-crosslinked Gelatin Conduit Promotes Regeneration after Peripheral Nerve Injury in Adult Rats
- PMID: 29235541
- PMCID: PMC5727525
- DOI: 10.1038/s41598-017-17792-2
Biodegradable Bisvinyl Sulfonemethyl-crosslinked Gelatin Conduit Promotes Regeneration after Peripheral Nerve Injury in Adult Rats
Abstract
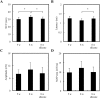
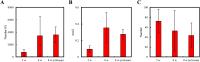
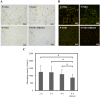
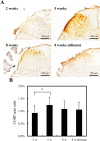
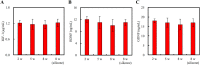
In our previous study, we found that gelatin-based materials exhibit good conductivity and are non-cytotoxic. In this study, gelatin was cross-linked with bisvinyl sulfonemethyl (BVSM) to fabricate a biodegradable conduit for peripheral nerve repair. First, BVSM on the prepared conduit was characterized to determine its mechanical properties and contact angle. The maximum tensile strength and water contact angle of the gelatin-BVSM conduits were 23 ± 4.8 MPa and 74.7 ± 9°, which provided sufficient mechanical strength to resist muscular contraction; additionally, the surface was hydrophilic. Cytotoxicity and apoptosis assays using Schwann cells demonstrated that the gelatin-BVSM conduits are non-cytotoxic. Next, we examined the neuronal electrophysiology, animal behavior, neuronal connectivity, macrophage infiltration, calcitonin gene-related peptide localization and expression, as well as the expression levels of nerve regeneration-related proteins. The number of fluorogold-labelled cells and histological analysis of the gelatin-BVSM nerve conduits was similar to that observed with the clinical use of silicone rubber conduits after 8 weeks of repair. Therefore, our results demonstrate that gelatin-BVSM conduits are promising substrates for application as bioengineered grafts for nerve tissue regeneration.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

