microRNAs in Parkinson's Disease: From Pathogenesis to Novel Diagnostic and Therapeutic Approaches
- PMID: 29236052
- PMCID: PMC5751299
- DOI: 10.3390/ijms18122698
microRNAs in Parkinson's Disease: From Pathogenesis to Novel Diagnostic and Therapeutic Approaches
Abstract
Parkinson's disease (PD) is the most prevalent central nervous system (CNS) movement disorder and the second most common neurodegenerative disease overall. PD is characterized by the progressive loss of dopaminergic (DAergic) neurons in the substantia nigra pars compacta (SNpc) within the midbrain, accumulation of alpha-synuclein (α-SYN) in Lewy bodies and neurites and excessive neuroinflammation. The neurodegenerative processes typically begin decades before the appearance of clinical symptoms. Therefore, the diagnosis is achievable only when the majority of the relevant DAergic neurons have already died and for that reason available treatments are only palliative at best. The causes and mechanism(s) of this devastating disease are ill-defined but complex interactions between genetic susceptibility and environmental factors are considered major contributors to the etiology of PD. In addition to the role of classical gene mutations in PD, the importance of regulatory elements modulating gene expression has been increasingly recognized. One example is the critical role played by microRNAs (miRNAs) in the development and homeostasis of distinct populations of neurons within the CNS and, in particular, in the context of PD. Recent reports demonstrate how distinct miRNAs are involved in the regulation of PD genes, whereas profiling approaches are unveiling variations in the abundance of certain miRNAs possibly relevant either to the onset or to the progression of the disease. In this review, we provide an overview of the miRNAs recently found to be implicated in PD etiology, with particular focus on their potential relevance as PD biomarkers, as well as their possible use in PD targeted therapy.
Keywords: biomarkers; exosomes; microRNAs; neuroprotective therapies; parkinson’s disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
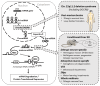
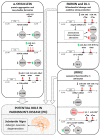

References
-
- Dorsey E.R., Constantinescu R., Thompson J.P., Biglan K.M., Holloway R.G., Kieburtz K., Marshall F.J., Ravina B.M., Schifitto G., Siderowf A., et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. - DOI - PubMed
-
- Fahn S., Elton R.L. UPDRS Development Committee, “The Unified Parkinson’s Disease Rating Scale”. In: Fahn S., Marsden C.D., Calne D.B., Goldstein M., editors. Recent Developments in Parkinson’s Disease. 2nd ed. Macmillan Healthcare Information; Florham Park, NJ, USA: 1987. pp. 153–163.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

