Recent Advances in Enzymatic Complexity Generation: Cyclization Reactions
- PMID: 29236467
- PMCID: PMC5988943
- DOI: 10.1021/acs.biochem.7b01161
Recent Advances in Enzymatic Complexity Generation: Cyclization Reactions
Abstract
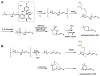
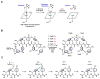
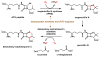
Enzymes in biosynthetic pathways, especially in plant and microbial metabolism, generate structural and functional group complexity in small molecules by conversion of acyclic frameworks to cyclic scaffolds via short, efficient routes. The distinct chemical logic used by several distinct classes of cyclases, oxidative and non-oxidative, has recently been elucidated by genome mining, heterologous expression, and genetic and mechanistic analyses. These include enzymes performing pericyclic transformations, pyran synthases, tandem acting epoxygenases, and epoxide "hydrolases", as well as oxygenases and radical S-adenosylmethionine enzymes that involve rearrangements of substrate radicals under aerobic or anaerobic conditions.
Figures

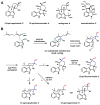
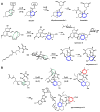



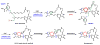


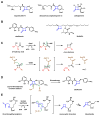





References
-
- Walsh CT, Tang Y. Natural Product Biosynthesis: Chemical Logic and Enzymatic Machinery. Royal Society of Chemistry; Crodon, UK: 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

