Impaired cardiac contractile function in arginine:glycine amidinotransferase knockout mice devoid of creatine is rescued by homoarginine but not creatine
- PMID: 29236952
- PMCID: PMC5982714
- DOI: 10.1093/cvr/cvx242
Impaired cardiac contractile function in arginine:glycine amidinotransferase knockout mice devoid of creatine is rescued by homoarginine but not creatine
Abstract
Aims: Creatine buffers cellular adenosine triphosphate (ATP) via the creatine kinase reaction. Creatine levels are reduced in heart failure, but their contribution to pathophysiology is unclear. Arginine:glycine amidinotransferase (AGAT) in the kidney catalyses both the first step in creatine biosynthesis as well as homoarginine (HA) synthesis. AGAT-/- mice fed a creatine-free diet have a whole body creatine-deficiency. We hypothesized that AGAT-/- mice would develop cardiac dysfunction and rescue by dietary creatine would imply causality.
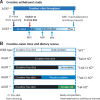
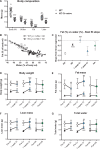
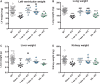
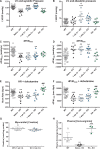
Methods and results: Withdrawal of dietary creatine in AGAT-/- mice provided an estimate of myocardial creatine efflux of ∼2.7%/day; however, in vivo cardiac function was maintained despite low levels of myocardial creatine. Using AGAT-/- mice naïve to dietary creatine we confirmed absence of phosphocreatine in the heart, but crucially, ATP levels were unchanged. Potential compensatory adaptations were absent, AMPK was not activated and respiration in isolated mitochondria was normal. AGAT-/- mice had rescuable changes in body water and organ weights suggesting a role for creatine as a compatible osmolyte. Creatine-naïve AGAT-/- mice had haemodynamic impairment with low LV systolic pressure and reduced inotropy, lusitropy, and contractile reserve. Creatine supplementation only corrected systolic pressure despite normalization of myocardial creatine. AGAT-/- mice had low plasma HA and supplementation completely rescued all other haemodynamic parameters. Contractile dysfunction in AGAT-/- was confirmed in Langendorff perfused hearts and in creatine-replete isolated cardiomyocytes, indicating that HA is necessary for normal cardiac function.
Conclusions: Our findings argue against low myocardial creatine per se as a major contributor to cardiac dysfunction. Conversely, we show that HA deficiency can impair cardiac function, which may explain why low HA is an independent risk factor for multiple cardiovascular diseases.
© The Author 2017 Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures



References
-
- Neubauer S. The failing heart – an engine out of fuel. N Engl J Med 2007;356:1140–1151. - PubMed
-
- Wyss M, Kaddurah-Daouk R.. Creatine and creatinine metabolism. Physiol Rev 2000;80:1107–1213. - PubMed
-
- Lygate CA, Fischer A, Sebag-Montefiore L, Wallis J, Ten Hove M, Neubauer S.. The creatine kinase energy transport system in the failing mouse heart. J Mol Cell Cardiol 2007;42:1129–1136. - PubMed
-
- Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K.. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 1997;96:2190–2196. - PubMed
-
- Lorentzon M, Ramunddal T, Bollano E, Soussi B, Waagstein F, Omerovic E.. In vivo effects of myocardial creatine depletion on left ventricular function, morphology, and energy metabolism-consequences in acute myocardial infarction. J Card Fail 2007;13:230–237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

