Kallikrein Cleaves C3 and Activates Complement
- PMID: 29237166
- PMCID: PMC6757171
- DOI: 10.1159/000484257
Kallikrein Cleaves C3 and Activates Complement
Abstract
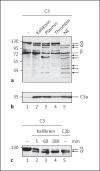
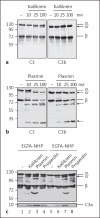
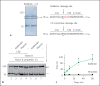
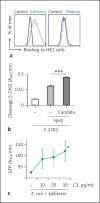
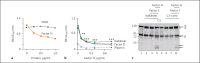
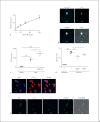
The human plasma contact system is an immune surveillance system activated by the negatively charged surfaces of bacteria and fungi and includes the kallikrein-kinin, the coagulation, and the fibrinolytic systems. Previous work shows that the contact system also activates complement, and that plasma enzymes like kallikrein, plasmin, thrombin, and FXII are involved in the activation process. Here, we show for the first time that kallikrein cleaves the central complement component C3 directly to yield active components C3b and C3a. The cleavage site within C3 is identical to that recognized by the C3 convertase. Also, kallikrein-generated C3b forms C3 convertases, which trigger the C3 amplification loop. Since kallikrein also cleaves factor B to yield Bb and Ba, kallikrein alone can trigger complement activation. Kallikrein-generated C3 convertases are inhibited by factor H; thus, the kallikrein activation pathway merges with the amplification loop of the alternative pathway. Taken together, these data suggest that activation of the contact system locally enhances complement activation on cell surfaces. The human pathogenic microbe Candida albicans activates the contact system in normal human serum. However, C. albicans immediately recruits factor H to the surface, thereby evading the alternative and likely kallikrein-mediated complement pathways.
Keywords:
© 2017 S. Karger AG, Basel.
Figures

References
-
- Nickel KF, Renné T. Crosstalk of the plasma contact system with bacteria. Thromb Res. 2012;130:S78–S83. - PubMed
-
- Maas C, Oschatz C, Renné T. The plasma contact system 2.0. Semin Thromb Hemost. 2011;37:375–381. - PubMed
-
- Zerleder S. C1-inhibitor: more than a serine protease inhibitor. Semin Thromb Hemost. 2011;37:362–374. - PubMed
-
- Derkx FH, Bouma BN, Schalekamp MP, Schalekamp MA. An intrinsic factor XII- prekallikrein-dependent pathway activates the human plasma renin-angiotensin system. Nature. 1979;280:315–316. - PubMed
-
- Ekdahl KN, Teramura Y, Hamad OA, Asif S, Duehrkop C, Fromell K, Gustafson E, Hong J, Kozarcanin H, Magnusson PU, Huber-Lang M, Garred P, Nilsson B. Dangerous liaisons: complement, coagulation, and kallikrein/kinin cross-talk act as a linchpin in the events leading to thromboinflammation. Immunol Rev. 2016;274:245–269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

