Sex steroid hormones and brain function: PET imaging as a tool for research
- PMID: 29237239
- PMCID: PMC5838537
- DOI: 10.1111/jne.12565
Sex steroid hormones and brain function: PET imaging as a tool for research
Abstract
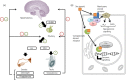
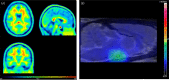
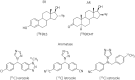
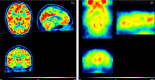
Sex steroid hormones are major regulators of sexual characteristic among species. These hormones, however, are also produced in the brain. Steroidal hormone-mediated signalling via the corresponding hormone receptors can influence brain function at the cellular level and thus affect behaviour and higher brain functions. Altered steroid hormone signalling has been associated with psychiatric disorders, such as anxiety and depression. Neurosteroids are also considered to have a neuroprotective effect in neurodegenerative diseases. So far, the role of steroid hormone receptors in physiological and pathological conditions has mainly been investigated post mortem on animal or human brain tissues. To study the dynamic interplay between sex steroids, their receptors, brain function and behaviour in psychiatric and neurological disorders in a longitudinal manner, however, non-invasive techniques are needed. Positron emission tomography (PET) is a non-invasive imaging tool that is used to quantitatively investigate a variety of physiological and biochemical parameters in vivo. PET uses radiotracers aimed at a specific target (eg, receptor, enzyme, transporter) to visualise the processes of interest. In this review, we discuss the current status of the use of PET imaging for studying sex steroid hormones in the brain. So far, PET has mainly been investigated as a tool to measure (changes in) sex hormone receptor expression in the brain, to measure a key enzyme in the steroid synthesis pathway (aromatase) and to evaluate the effects of hormonal treatment by imaging specific downstream processes in the brain. Although validated radiotracers for a number of targets are still warranted, PET can already be a useful technique for steroid hormone research and facilitate the translation of interesting findings in animal studies to clinical trials in patients.
Keywords: androgen receptor; neuroimaging; oestrogen receptor; positron emission tomography; sex steroid hormones.
© 2017 The Authors. Journal of Neuroendocrinology published by John Wiley & Sons Ltd on behalf of British Society for Neuroendocrinology.
Figures
References
-
- Simerly RB. Wired on hormones: endocrine regulation of hypothalamic development. Curr Opin Neurobiol. 2005;15:81‐85. - PubMed
-
- Diamanti‐Kandarakis E, Dattilo M, Macut D, et al. Mechanisms in endocrinology : aging and anti‐aging: a combo‐endocrinology overview. Eur J Endocrinol 2017;176:R283‐R308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

