Adolescent Coordinated Transition (ACT) to improve health outcomes among young people living with HIV in Nigeria: study protocol for a randomized controlled trial
- PMID: 29237487
- PMCID: PMC5729403
- DOI: 10.1186/s13063-017-2347-z
Adolescent Coordinated Transition (ACT) to improve health outcomes among young people living with HIV in Nigeria: study protocol for a randomized controlled trial
Erratum in
-
Correction to: Adolescent Coordinated Transition (ACT) to improve health outcomes among young people living with HIV in Nigeria: study protocol for a randomized controlled trial.Trials. 2018 Feb 13;19(1):104. doi: 10.1186/s13063-018-2463-4. Trials. 2018. PMID: 29439727 Free PMC article.
Abstract
Background: Adolescents living with HIV (ALHIV) have worse health outcomes than other populations of people living with HIV. Contributing factors include lack of standard and comprehensive procedures for ALHIV transitioning from pediatric to adult care. This has contributed to poor retention at, and following transition, which is problematic especially in high ALHIV-burden, resource-limited settings like Nigeria.
Methods: Using a two-arm cluster randomized control design, the Adolescent Coordinated Transition (ACT) trial will measure the comparative effectiveness of a graduated transition and organized support group intervention against the usual practice of abrupt transfer of Nigerian ALHIV from pediatric to adult care. This study will be conducted at 12 secondary and tertiary healthcare facilities (six intervention, six control) across all six of Nigeria's geopolitical zones. The study population is 13- to 17-year-old ALHIV (N = 216, n = 108 per study arm) on antiretroviral therapy. Study participants will be followed through a 12-month pre-transfer/transition period and for an additional 24 months post transfer/transition. The primary outcome measure is the proportion of ALHIV retained in care at 12 and 24 months post transfer. Secondary outcome measures are proportions of ALHIV achieving viral suppression and demonstrating increased psychosocial wellbeing and self-efficacy measured by psychometric tests including health locus of control, functional social support, perceived mental health, and sexual risk and behavior.
Discussion: We hypothesize that the ACT intervention will significantly increase psychosocial wellbeing, retention in care and ultimately viral suppression among ALHIV. ACT's findings have the potential to facilitate the development of standard guidelines for transitioning ALHIV and improving health outcomes in this population. The engagement of a consortium of local implementing partners under the Nigeria Implementation Science Alliance allows for nationwide study implementation and expedient results dissemination to program managers and policy-makers. Ultimately, ACT may also provide evidence to inform transitioning guidelines not only for ALHIV but for adolescents living with other chronic diseases in resource-limited settings.
Trial registration: ClinicalTrials.gov, ID: NCT03152006 . Registered on May 12, 2017.
Keywords: Adolescent; HIV; Healthcare transition; Mental health; Nigeria; Retention; Viral suppression.
Conflict of interest statement
Ethics approvals and consent to participate
The ACT trial has been approved by the Institutional Review Boards (IRBs) of the University of Nevada Las Vegas (#990070-4) and the University of Maryland (#HP-00075335), as well as the Nigerian National Health Research Ethics Committee (NHREC/01/01/2007-25/04/2017). Written informed consent will be obtained from parents/guardians and eligible emancipated minor ALHIV, and assent will be obtained from all eligible minor ALHIV. All IRBs will be notified of any modifications to the protocol.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
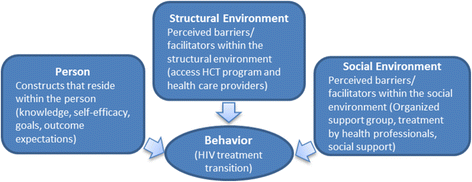
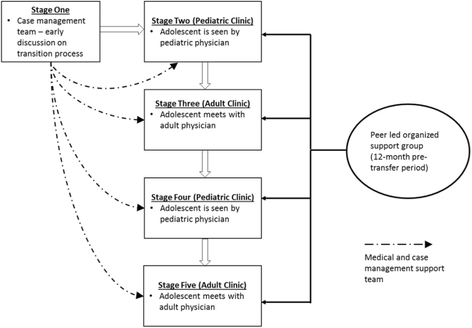
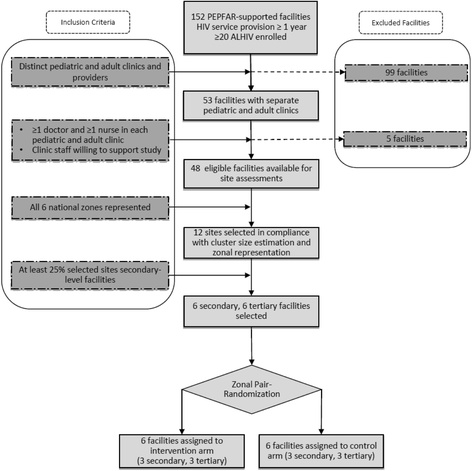
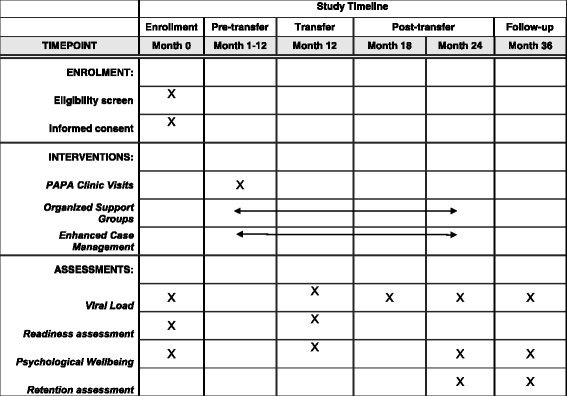
References
-
- UNAIDS. Report on the Global AIDS Epidemic 2013. 2013. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/ epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. 2016.
-
- UNAIDS, “HIV indicators,” AIDSinfo 2016 Data, 2017. [Online]. Available at: http://aidsinfo.unaids.org/.
-
- World Health Organization. Health for the world’s adolescents: a second chance in the second decade. 2014 Available at: http://apps.who.int/iris/bitstream/10665/112750/1/WHO_FWC_MCA_14.05_eng..... 2015.
-
- World Health Organization. Care and treatment values, preferences, and attitudes of adolescents living with HIV: a review of qualitative literature. 2013. Available at: http://apps.who.int/iris/bitstream/10665/95145/1/WHO_HIV_2013.137_eng.pdf. 2015.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

