Nucleic Acid Amplification Test Quantitation as Predictor of Toxin Presence in Clostridium difficile Infection
- PMID: 29237788
- PMCID: PMC5824036
- DOI: 10.1128/JCM.01316-17
Nucleic Acid Amplification Test Quantitation as Predictor of Toxin Presence in Clostridium difficile Infection
Abstract
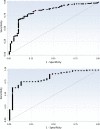
Multistep algorithmic testing in which a sensitive nucleic acid amplification test (NAAT) is followed by a specific toxin A and toxin B enzyme immunoassay (EIA) is among the most accurate methods for Clostridium difficile infection (CDI) diagnosis. The obvious shortcoming of this approach is that multiple tests must be performed to establish a CDI diagnosis, which may delay treatment. Therefore, we sought to determine whether a preliminary diagnosis could be made on the basis of the quantitative results of the first test in algorithmic testing, which provide a measure of organism burden. To do so, we retrospectively analyzed two large collections of samples (n = 2,669 and n = 1,718) that were submitted to the laboratories of two Dutch hospitals for CDI testing. Both hospitals apply a two-step testing algorithm in which a NAAT is followed by a toxin A/B EIA. Of all samples, 208 and 113 samples, respectively, tested positive by NAAT. Among these NAAT-positive samples, significantly lower mean quantification cycle (Cq ) values were found for patients whose stool eventually tested positive for toxin, compared with patients who tested negative for toxin (mean Cq values of 24.4 versus 30.4 and 26.8 versus 32.2; P < 0.001 for both cohorts). Receiver operating characteristic curve analysis was performed to investigate the ability of Cq values to predict toxin status and yielded areas under the curve of 0.826 and 0.854. Using the optimal Cq cutoff values, prediction of the eventual toxin A/B EIA results was accurate for 78.9% and 80.5% of samples, respectively. In conclusion, Cq values can serve as predictors of toxin status but, due to the suboptimal correlation between the two tests, additional toxin testing is still needed.
Keywords: Clostridium difficile; NAAT quantitation; diagnostic.
Copyright © 2018 American Society for Microbiology.
Figures

Similar articles
-
Comparison of Clostridioides difficile Stool Toxin Concentrations in Adults With Symptomatic Infection and Asymptomatic Carriage Using an Ultrasensitive Quantitative Immunoassay.Clin Infect Dis. 2019 Jan 1;68(1):78-86. doi: 10.1093/cid/ciy415. Clin Infect Dis. 2019. PMID: 29788296 Free PMC article.
-
Can a toxin gene NAAT be used to predict toxin EIA and the severity of Clostridium difficile infection?Antimicrob Resist Infect Control. 2017 Dec 19;6:127. doi: 10.1186/s13756-017-0283-z. eCollection 2017. Antimicrob Resist Infect Control. 2017. PMID: 29270290 Free PMC article.
-
Diagnosis and outcome of Clostridium difficile infection by toxin enzyme immunoassay and polymerase chain reaction in an island population.J Gastroenterol Hepatol. 2017 Feb;32(2):415-419. doi: 10.1111/jgh.13504. J Gastroenterol Hepatol. 2017. PMID: 27505006
-
A Laboratory Medicine Best Practices Systematic Review and Meta-analysis of Nucleic Acid Amplification Tests (NAATs) and Algorithms Including NAATs for the Diagnosis of Clostridioides (Clostridium) difficile in Adults.Clin Microbiol Rev. 2019 May 29;32(3):e00032-18. doi: 10.1128/CMR.00032-18. Print 2019 Jun 19. Clin Microbiol Rev. 2019. PMID: 31142497 Free PMC article.
-
Diagnostic pitfalls in Clostridium difficile infection.Infect Dis Clin North Am. 2015 Mar;29(1):63-82. doi: 10.1016/j.idc.2014.11.008. Epub 2015 Jan 13. Infect Dis Clin North Am. 2015. PMID: 25595842 Review.
Cited by
-
Prospective Evaluation of the mariPOC Test for Detection of Clostridioides difficile Glutamate Dehydrogenase and Toxins A/B.J Clin Microbiol. 2020 Mar 25;58(4):e01872-19. doi: 10.1128/JCM.01872-19. Print 2020 Mar 25. J Clin Microbiol. 2020. PMID: 31941691 Free PMC article.
-
Laboratory Tests for the Diagnosis of Clostridium difficile.Clin Colon Rectal Surg. 2020 Mar;33(2):73-81. doi: 10.1055/s-0039-3400476. Epub 2020 Feb 25. Clin Colon Rectal Surg. 2020. PMID: 32104159 Free PMC article. Review.
-
The predictive value of quantitative nucleic acid amplification detection of Clostridium difficile toxin gene for faecal sample toxin status and patient outcome.PLoS One. 2018 Dec 5;13(12):e0205941. doi: 10.1371/journal.pone.0205941. eCollection 2018. PLoS One. 2018. PMID: 30517094 Free PMC article. Clinical Trial.
-
Ultrasensitive Detection of Clostridioides difficile Toxins A and B by Use of Automated Single-Molecule Counting Technology.J Clin Microbiol. 2018 Oct 25;56(11):e00908-18. doi: 10.1128/JCM.00908-18. Print 2018 Nov. J Clin Microbiol. 2018. PMID: 30158195 Free PMC article.
-
Composition of gut microbiota in patients with toxigenic Clostridioides (Clostridium) difficile: Comparison between subgroups according to clinical criteria and toxin gene load.PLoS One. 2019 Feb 20;14(2):e0212626. doi: 10.1371/journal.pone.0212626. eCollection 2019. PLoS One. 2019. PMID: 30785932 Free PMC article.
References
-
- Leffler DA, Lamont JT. 2015. Clostridium difficile infection. N Engl J Med 373:287–288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

