Determinants of exacerbation risk in patients with COPD in the TIOSPIR study
- PMID: 29238184
- PMCID: PMC5713692
- DOI: 10.2147/COPD.S145814
Determinants of exacerbation risk in patients with COPD in the TIOSPIR study
Abstract
Background: Exacerbation history is used to grade the risk of COPD exacerbation, but its reliability and relationship to other risk factors and prior therapy is unclear. To examine these interrelationships, we conducted a post hoc analysis of patients in the TIOSPIR trial with ≥2 years' follow-up or who died on treatment.
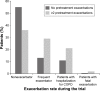
Patients and methods: Patients were grouped by their annual exacerbation rate on treatment into nonexacerbators, infrequent, and frequent exacerbators (annual exacerbation rates 0, ≤1, and >1, respectively), and baseline characteristics discriminating among the groups were determined. We used univariate and multivariate analyses to explore the effect of baseline characteristics on risk of exacerbation, hospitalization (severe exacerbation), and death (all causes).
Results: Of 13,591 patients, 6,559 (48.3%) were nonexacerbators, 4,568 (33.6%) were infrequent exacerbators, and 2,464 (18.1%) were frequent exacerbators; 45% of patients without exacerbations in the previous year exacerbated on treatment. Multivariate analysis identified baseline pulmonary maintenance medication as a predictive factor of increased exacerbation risk, with inhaled corticosteroid treatment associated with increased exacerbation risk irrespective of exacerbation history.
Conclusion: Our data confirm established risk factors for exacerbation, but highlight the limitations of exacerbation history when categorizing patients and the importance of prior treatment when identifying exacerbation risk.
Keywords: COPD; exacerbation; frequent exacerbators.
Conflict of interest statement
Disclosure PMAC reports receiving consulting fees, lecture fees, and travel support from Novartis, GlaxoSmithKline, Boehringer Ingelheim, and Takeda. DD reports receiving consulting fees, lecture fees, and payment for the development of educational activities from Boehringer Ingelheim, Pfizer, Novartis, Chiesi, Nycomed, and Dey Pharma. RAW reports receiving consulting fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, ContraFect, GlaxoSmithKline, Janssen, Mylan, Novartis, Pfizer, Pulmonx, Roche, Spiration, Sunovion, Teva, Theravance, Verona, and Vertex, and grant support from Boehringer Ingelheim, Glaxo-SmithKline, and Pearl Therapeutics. KT, AM, and NM are employees of Boehringer Ingelheim, and ARA reports receiving consulting fees, lecture fees, and travel support from AstraZeneca, Boehringer Ingelheim, Forest Laboratories, GlaxoSmithKline, and Novartis and grant support from GlaxoSmithKline. The authors report no other conflicts of interest in this work.
Figures


References
-
- Vestbo J, Edwards LD, Scanlon PD, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184–1192. - PubMed
-
- Wilke S, Jones PW, Müllerova H, et al. One-year change in health status and subsequent outcomes in COPD. Thorax. 2015;70(5):420–425. - PubMed
-
- Müllerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147(4):999–1007. - PubMed
-
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

