Role of Metcalfa pruinosa as a Vector for Pseudomonas syringae pv. actinidiae
- PMID: 29238278
- PMCID: PMC5720602
- DOI: 10.5423/PPJ.OA.04.2017.0074
Role of Metcalfa pruinosa as a Vector for Pseudomonas syringae pv. actinidiae
Abstract
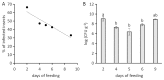
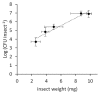
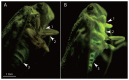
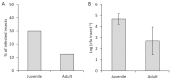
After 20 years of steady increase, kiwifruit industry faced a severe arrest due to the pandemic spread of the bacterial canker, caused by Pseudomonas syringae pv. actinidiae (Psa). The bacterium penetrates the host plant primarily via natural openings or wounds, and its spread is mainly mediated by atmospheric events and cultural activities. Since the role of sucking insects as vectors of bacterial pathogens is widely documented, we investigated the ability of Metcalfa pruinosa Say (1830), one of the most common kiwifruit pests, to transmit Psa to healthy plants in laboratory conditions. Psa could be isolated both from insects feeding over experimentally inoculated plants, and from insects captured in Psa-infected orchards. Furthermore, insects were able to transmit Psa from experimentally inoculated plants to healthy ones. In conclusion, the control of M. pruinosa is recommended in the framework of protection strategies against Psa.
Keywords: bacterial canker of kiwifruit; insect-mediated disease transmission; microscopical visualization.
Figures

References
-
- Agrios GN. Transmission of plant disease by insects. In: Capinera JL, editor. Encyclopedia of entomology. Springer; Berlin, Germany: 2008. pp. 2290–2317.
-
- Brown C, Lynch L, Zilberman D. The economics of controlling insect-transmitted plant diseases. Am J Agric Econ. 2002;84:279–291. doi: 10.1111/1467-8276.00297. - DOI
-
- Chiykowski LN, Sinha RC. Some factors affecting the transmission of eastern peach X-mycoplasmalike organism by the leafhopper Paraphlepsius irroratus. Can J Plant Pathol. 1988;10:85–92. doi: 10.1080/07060668809501738. - DOI
-
- Donati I, Buriani G, Cellini A, Mauri S, Costa G, Spinelli F. New insights on the bacterial canker of kiwifruit (Pseudomonas syringae pv. actinidiae) J Berry Res. 2014;4:53–67. doi: 10.3233/JBR-140073. - DOI
-
- FAO. [30 March 2017];FAOStat data on world kiwifruit production. URL http://www.fao.org/faostat/en/#data/QC/
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

