Rhizosphere Microbiome Recruited from a Suppressive Compost Improves Plant Fitness and Increases Protection against Vascular Wilt Pathogens of Tomato
- PMID: 29238353
- PMCID: PMC5712882
- DOI: 10.3389/fpls.2017.02022
Rhizosphere Microbiome Recruited from a Suppressive Compost Improves Plant Fitness and Increases Protection against Vascular Wilt Pathogens of Tomato
Abstract
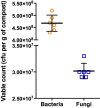
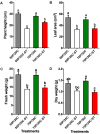
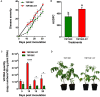
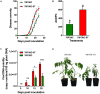
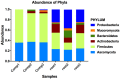
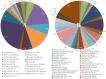
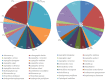
Suppressive composts represent a sustainable approach to combat soilborne plant pathogens and an alternative to the ineffective chemical fungicides used against those. Nevertheless, suppressiveness to plant pathogens and reliability of composts are often inconsistent with unpredictable effects. While suppressiveness is usually attributed to the compost's microorganisms, the mechanisms governing microbial recruitment by the roots and the composition of selected microbial communities are not fully elucidated. Herein, the purpose of the study was to evaluate the impact of a compost on tomato plant growth and its suppressiveness against Fusarium oxysporum f. sp. lycopersici (Foxl) and Verticillium dahliae (Vd). First, growth parameters of tomato plants grown in sterile peat-based substrates including 20 and 30% sterile compost (80P/20C-ST and 70P/30C-ST) or non-sterile compost (80P/20C and 70P/30C) were evaluated in a growth room experiment. Plant height, total leaf surface, and fresh and dry weight of plants grown in the non-sterile compost mixes were increased compared to the plants grown in the sterile compost substrates, indicating the plant growth promoting activity of the compost's microorganisms. Subsequently, compost's suppressiveness against Foxl and Vd was evaluated with pathogenicity experiments on tomato plants grown in 70P/30C-ST and 70P/30C substrates. Disease intensity was significantly less in plants grown in the non-sterile compost than in those grown in the sterile compost substrate; AUDPC was 2.3- and 1.4-fold less for Foxl and Vd, respectively. Moreover, fungal quantification in planta demonstrated reduced colonization in plants grown in the non-sterile mixture. To further investigate these findings, we characterized the culturable microbiome attracted by the roots compared to the unplanted compost. Bacteria and fungi isolated from unplanted compost and the rhizosphere of plants were sequence-identified. Community-level analysis revealed differential microbial communities between the compost and the rhizosphere, suggesting a clear effect of the plant in the microbiome assembly. Proteobacteria and Actinobacteria were highly enriched in the rhizosphere whereas Firmicutes were strongly represented in both compartments with Bacillus being the most abundant species. Our results shed light on the composition of a microbial consortium that could protect plants against the wilt pathogens of tomato and improve plant overall health.
Keywords: Fusarium oxysporum; Verticillium dahliae; compost; disease suppression; microbiome; plant growth promotion; rhizosphere.
Figures

References
-
- Angelopoulou D. J., Naska E. J., Paplomatas E. J., Tjamos S. E. (2014). Biological control agents (BCAs) of verticillium wilt: influence of application rates and delivery method on plant protection, triggering of host defence mechanisms and rhizosphere populations of BCAs. Plant Pathol. 63 k1062–1069. 10.1111/ppa.12198 - DOI
-
- Badri D. V., Chaparro J. M., Zhang R., Shen Q., Vivanco J. M. (2013). Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J. Biol. Chem. 288 4502–4512. 10.1074/jbc.M112.433300 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

