A feasibility study of neoadjuvant talazoparib for operable breast cancer patients with a germline BRCA mutation demonstrates marked activity
- PMID: 29238749
- PMCID: PMC5719044
- DOI: 10.1038/s41523-017-0052-4
A feasibility study of neoadjuvant talazoparib for operable breast cancer patients with a germline BRCA mutation demonstrates marked activity
Abstract
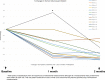
This study was undertaken to determine the feasibility of enrolling breast cancer patients on a single-agent-targeted therapy trial before neoadjuvant chemotherapy. Specifically, we evaluated talazoparib in patients harboring a deleterious BRCA mutation (BRCA+). Patients with a germline BRCA mutation and ≥1 cm, HER2-negative primary tumors were eligible. Study participants underwent a pretreatment biopsy, 2 months of talazoparib, off-study core biopsy, anthracycline, and taxane-based chemotherapy ± carboplatin, followed by surgery. Volumetric changes in tumor size were determined by ultrasound at 1 and 2 months of therapy. Success was defined as 20 patients accrued within 2 years and <33% experienced a grade 4 toxicity. The study was stopped early after 13 patients (BRCA1 + n = 10; BRCA2 + n = 3) were accrued within 8 months with no grade 4 toxicities and only one patient requiring dose reduction due to grade 3 neutropenia. The median age was 40 years (range 25-55) and clinical stage included I (n = 2), II (n = 9), and III (n = 2). Most tumors (n = 9) were hormone receptor-negative, and one of these was metaplastic. Decreases in tumor volume occurred in all patients following 2 months of talazoparib; the median was 88% (range 30-98%). Common toxicities were neutropenia, anemia, thrombocytopenia, nausea, dizziness, and fatigue. Single-agent-targeted therapy trials are feasible in BRCA+ patients. Given the rapid rate of accrual, profound response and favorable toxicity profile, the feasibility study was modified into a phase II study to determine pathologic complete response rates after 4-6 months of single-agent talazoparib.
Conflict of interest statement
Dr. Litton has clinical research to her Institution with Medivation/Pfizer, Astra-Zeneca, Genentech, Glaxo-Smith Kline, Novartis and served on Trial Steering Committees with Medivation/Pfizer and Novartis. Dr. Arun has clinical research with AbbVie and serves on a Trial Steering Committee for AbbVie. Gary Whitman owns stock in Pfizer, which is disclosed and within the limits of the MD Anderson conflict of interest policy. The remaining authors declare no competing financial interests.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous