Efficacy of High-Sensitivity Troponin T in Identifying Very-Low-Risk Patients With Possible Acute Coronary Syndrome
- PMID: 29238804
- PMCID: PMC5838590
- DOI: 10.1001/jamacardio.2017.4625
Efficacy of High-Sensitivity Troponin T in Identifying Very-Low-Risk Patients With Possible Acute Coronary Syndrome
Erratum in
-
Error in Methods Section.JAMA Cardiol. 2018 Sep 1;3(9):898. doi: 10.1001/jamacardio.2018.2503. JAMA Cardiol. 2018. PMID: 30090946 Free PMC article. No abstract available.
Abstract
Importance: Physicians need information on how to use the first available high-sensitivity troponin (hsTnT) assay in the United States to identify patients at very low risk for 30-day adverse cardiac events (ACE).
Objective: To determine whether a negative hsTnT assay at 0 and 3 hours following emergency department presentation could identify patients at less than 1% risk of a 30-day ACE.
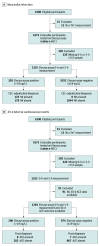
Design, setting, and participants: A prospective, observational study at 15 emergency departments in the United States between 2011 and 2015 that included individuals 21 years and older, presenting to the emergency department with suspected acute coronary syndrome. Of 1690 eligible individuals, 15 (no cardiac troponin T measurement) and 320 (missing a 0-hour or 3-hour sample) were excluded from the analyses.
Exposures: Serial hsTnT measurements (fifth-generation Roche Elecsys hsTnT assay).
Main outcomes and measures: Serial blood samples from each patient were collected after emergency department presentation (once identified as a potential patient with acute coronary syndrome) and 3 hours, 6 to 9 hours, and 12 to 24 hours later. Adverse cardiac events were defined as myocardial infarction, urgent revascularization, or death. The upper reference level for the hsTnT assay, defined as the 99th percentile, was established as 19 ng/L in a separate healthy US cohort. Patients were considered ruled out for acute myocardial infarction if their hsTnT level at 0 hours and 3 hours was less than the upper reference level. Gold standard diagnoses were determined by a clinical end point committee. Evaluation of assay clinical performance for acute myocardial infarction rule-out was prespecified; the hypothesis regarding 30-day ACE was formulated after data collection.
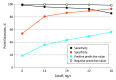
Results: In 1301 healthy volunteers (50.4% women; median age, 48 years), the upper reference level was 19 ng/L. In 1600 patients with suspected acute coronary syndrome (48.4% women; median age, 55 years), a single hsTnTlevel less than 6 ng/L at baseline had a negative predictive value for AMI of 99.4%. In 974 patients (77.1%) with both 0-hour and 3-hour hsTnT levels of 19 ng/L or less, the negative predictive value for 30-day ACE was 99.3% (95% CI, 99.1-99.6). Using sex-specific cutpoints, C statistics for women (0.952) and men (0.962) were similar for acute myocardial infarction.
Conclusions and relevance: A single hsTnT level less than 6 ng/L was associated with a markedly decreased risk of AMI, while serial levels at 19 ng/L or less identified patients at less than 1% risk of 30-day ACE.
Conflict of interest statement
Figures
Comment in
-
The Wait for High-Sensitivity Troponin Is Over-Proceed Cautiously.JAMA Cardiol. 2018 Feb 1;3(2):112-113. doi: 10.1001/jamacardio.2017.4626. JAMA Cardiol. 2018. PMID: 29238815 No abstract available.
References
-
- CDC . National hospital ambulatory medical care survey: 2010 emergency department summary tables. Washington, DC: Centers for Disease Control and Prevention, Ambulatory and Hospital Care Statistics Branch; 2010.
-
- Mitchell AM, Garvey JL, Chandra A, Diercks D, Pollack CV, Kline JA. Prospective multicenter study of quantitative pretest probability assessment to exclude acute coronary syndrome for patients evaluated in emergency department chest pain units. Ann Emerg Med. 2006;47(5):447. - PubMed
-
- Reilly BM, Evans AT, Schaider JJ, Wang Y. Triage of patients with chest pain in the emergency department: a comparative study of physicians’ decisions. Am J Med. 2002;112(2):95-103. - PubMed
-
- Freas GC. Medicolegal aspects of acute myocardial infarction. Emerg Med Clin North Am. 2001;19(2):511-521. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

