An electric-eel-inspired soft power source from stacked hydrogels
- PMID: 29239354
- PMCID: PMC6436395
- DOI: 10.1038/nature24670
An electric-eel-inspired soft power source from stacked hydrogels
Abstract
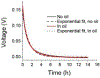
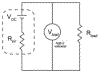
Progress towards the integration of technology into living organisms requires electrical power sources that are biocompatible, mechanically flexible, and able to harness the chemical energy available inside biological systems. Conventional batteries were not designed with these criteria in mind. The electric organ of the knifefish Electrophorus electricus (commonly known as the electric eel) is, however, an example of an electrical power source that operates within biological constraints while featuring power characteristics that include peak potential differences of 600 volts and currents of 1 ampere. Here we introduce an electric-eel-inspired power concept that uses gradients of ions between miniature polyacrylamide hydrogel compartments bounded by a repeating sequence of cation- and anion-selective hydrogel membranes. The system uses a scalable stacking or folding geometry that generates 110 volts at open circuit or 27 milliwatts per square metre per gel cell upon simultaneous, self-registered mechanical contact activation of thousands of gel compartments in series while circumventing power dissipation before contact. Unlike typical batteries, these systems are soft, flexible, transparent, and potentially biocompatible. These characteristics suggest that artificial electric organs could be used to power next-generation implant materials such as pacemakers, implantable sensors, or prosthetic devices in hybrids of living and non-living systems.
Conflict of interest statement
Author Information:
The authors declare no competing financial interests.
Figures
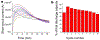



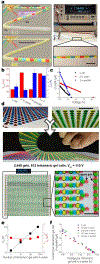

Comment in
-
Electric fish inspire inventors across the centuries.Nature. 2018 Mar 8;555(7695):165. doi: 10.1038/d41586-018-02832-2. Nature. 2018. PMID: 29517028 No abstract available.
References
-
- Bennett MVL Electric Organs in Fish Physiology (ed. Randall WSH. and D. J.) 5, 347–491 (Academic Press, 1971).
-
- Gotter AL, Kaetzel MA & Dedman JR Electrophorus electricus as a model system for the study of membrane excitability. Comp. Biochem. Physiol. A. Mol. Integr. Physiol 119, 225–241 (1998). - PubMed
-
- Sun H, Fu X, Xie S, Jiang Y & Peng H Electrochemical Capacitors with High Output Voltages that Mimic Electric Eels. Adv. Mater. 28, 2070–2076 (2016). - PubMed
-
- Kim D-H et al. Epidermal Electronics. Science 333, 838–843 (2011). - PubMed

