Fluorescence detection, enumeration and characterization of single circulating cells in vivo: technology, applications and future prospects
- PMID: 29240559
- PMCID: PMC5753400
- DOI: 10.1088/1361-6560/aa98f9
Fluorescence detection, enumeration and characterization of single circulating cells in vivo: technology, applications and future prospects
Abstract
There are many diseases and biological processes that involve circulating cells in the bloodstream, such as cancer metastasis, immunology, reproductive medicine, and stem cell therapies. This has driven significant interest in new technologies for the study of circulating cells in small animal research models and clinically. Most currently used methods require drawing and enriching blood samples from the body, but these suffer from a number of limitations. In contrast, 'in vivo flow cytometry' (IVFC) refers to set of technologies that allow study of cells directly in the bloodstream of the organism in vivo. In recent years the IVFC field has grown significantly and new techniques have been developed, including fluorescence microscopy, multi-photon, photo-acoustic, and diffuse fluorescence IVFC. In this paper we review recent technical advances in IVFC, with emphasis on instrumentation, contrast mechanisms, and detection sensitivity. We also describe key applications in biomedical research, including cancer research and immunology. Last, we discuss future directions for IVFC, as well as prospects for broader adoption by the biomedical research community and translation to humans clinically.
Figures

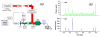


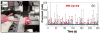


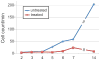

References
-
- Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, Brannigan BW, Kapur R, Stott SL, Shioda T, Ramaswamy S, Ting DT, Lin CP, Toner M, Haber DA, Maheswaran S. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–22. - PMC - PubMed
-
- Andreopoulou E, Yang LY, Rangel KM, Reuben JM, Hsu L, Krishnamurthy S, Valero V, Fritsche HA, Cristofanilli M. Comparison of assay methods for detection of circulating tumor cells in metastatic breast cancer: AdnaGen AdnaTest BreastCancer Select/Detect versus Veridex CellSearch system. Int J Cancer. 2012;130:1590–7. - PubMed
-
- Azab AK, Hu J, Quang P, Azab F, Pitsillides C, Awwad R, Thompson B, Maiso P, Sun JD, Hart CP, Roccaro AM, Sacco A, Ngo HT, Lin CP, Kung AL, Carrasco RD, Vanderkerken K, Ghobrial IM. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood. 2012;119:5782–94. - PMC - PubMed
-
- Bidard F-C, Peeters DJ, Fehm T, Nolé F, Gisbert-Criado R, Mavroudis D, Grisanti S, Generali D, Garcia-Saenz JA, Stebbing J, Caldas C, Gazzaniga P, Manso L, Zamarchi R, de Lascoiti AF, De Mattos-Arruda L, Ignatiadis M, Lebofsky R, van Laere SJ, Meier-Stiegen F, Sandri M-T, Vidal-Martinez J, Politaki E, Consoli F, Bottini A, Diaz-Rubio E, Krell J, Dawson S-J, Raimondi C, Rutten A, Janni W, Munzone E, Carañana V, Agelaki S, Almici C, Dirix L, Solomayer E-F, Zorzino L, Johannes H, Reis-Filho JS, Pantel K, Pierga J-Y, Michiels S. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. The Lancet Oncology. 15:406–14. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
