Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs
- PMID: 29240946
- PMCID: PMC5829639
- DOI: 10.1093/nar/gkx1239
Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs
Abstract
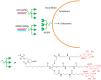
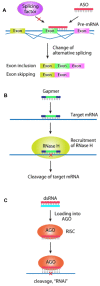
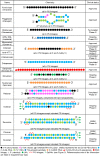
RNA plays a central role in the expression of all genes. Because any sequence within RNA can be recognized by complementary base pairing, synthetic oligonucleotides and oligonucleotide mimics offer a general strategy for controlling processes that affect disease. The two primary antisense approaches for regulating expression through recognition of cellular RNAs are single-stranded antisense oligonucleotides and duplex RNAs. This review will discuss the chemical modifications and molecular mechanisms that make synthetic nucleic acid drugs possible. Lessons learned from recent clinical trials will be summarized. Ongoing clinical trials are likely to decisively test the adequacy of our current generation of antisense nucleic acid technologies and highlight areas where more basic research is needed.
Figures



References
-
- Crooke S.T., Wang S., Vickers T.A., Shen W., Liang X.H.. Cellular uptake and trafficking of antisense oligonucleotides. Nat. Biotechnol. 2017; 35:230–237. - PubMed
-
- Deleavey G.F., Damha M.J.. Designing chemically modified oligonucleotides for targeted gene silencing. Chem. Biol. 2012; 19:937–954. - PubMed

