Anti-IgE treatment with oral immunotherapy in multifood allergic participants: a double-blind, randomised, controlled trial
- PMID: 29242014
- PMCID: PMC6944204
- DOI: 10.1016/S2468-1253(17)30392-8
Anti-IgE treatment with oral immunotherapy in multifood allergic participants: a double-blind, randomised, controlled trial
Abstract
Background: Despite progress in single food oral immunotherapy, there is little evidence concerning the safety and efficacy of treating individuals with multiple food (multifood) allergies. We did a pilot study testing whether anti-IgE (omalizumab) combined with multifood oral immunotherapy benefited multifood allergic patients.
Methods: We did a blinded, phase 2 clinical trial at Stanford University. We enrolled participants, aged 4-15 years, with multifood allergies validated by double-blind, placebo-controlled food challenges to their offending foods. Inclusion criteria included a positive skin prick test of 6 mm or more (wheal diameter, above the negative control), a food-specific serum IgE concentration of more than 4 kU/L for each food, or both, and a positive double-blind, placebo-controlled food challenge at 500 mg or less of food protein. Exclusion criteria included eosinophilic oesophagitis and severe asthma. Participants were randomised (3:1) with a block size of four, to receive multifood oral immunotherapy to two to five foods, together with omalizumab (n=36) or placebo (n=12). 12 individuals who fulfilled the same inclusion and exclusion criteria were included as controls. These individuals were not randomised and received neither omalizumab nor oral immunotherapy. Omalizumab or placebo was administered subcutaneously for 16 weeks, with oral immunotherapy starting at week 8, and was stopped 20 weeks before the exit double-blind, placebo-controlled food challenge at week 36. The primary endpoint was the proportion of participants who passed double-blind, placebo-controlled food challenges to at least two of their offending foods. This completed trial is registered with ClinicalTrials.gov, number NCT02643862.
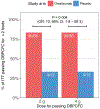
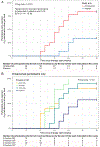
Findings: Between March 25, 2015, and Aug 18, 2016, 165 participants were assessed for eligibility, of whom 84 did not meet the inclusion criteria and 21 declined to participate. We enrolled and randomised 48 eligible participants and the remaining 12 patients were included as nonrandomised, untreated controls. At week 36, a significantly greater proportion of the omalizumab-treated (30 [83%] of 36) versus placebo (four [33%] of 12) participants passed double-blind, placebo-controlled food challenges to 2 g protein for two or more of their offending foods (odds ratio 10·0, 95% CI 1·8-58·3, p=0·0044). All participants completed the study. There were no serious or severe (grade 3 or worse) adverse events. Participants in the omalizumab group had a significantly lower median per-participant percentage of oral immunotherapy doses associated with any adverse events (27% vs 68%; p=0·0082). The most common adverse events in both groups were gastrointestinal events.
Interpretation: In multifood allergic patients, omalizumab improves the efficacy of multifood oral immunotherapy and enables safe and rapid desensitisation.
Funding: US National Institutes of Health (NIH).
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interest
We declare no competing interests.
Figures


Comment in
-
Food allergy: setting the scene for tolerance induction.Lancet Gastroenterol Hepatol. 2018 Feb;3(2):74-75. doi: 10.1016/S2468-1253(17)30391-6. Epub 2017 Dec 12. Lancet Gastroenterol Hepatol. 2018. PMID: 29242015 No abstract available.
Similar articles
-
Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): a large, randomised, double-blind, placebo-controlled, phase 2 study.Lancet. 2019 Oct 19;394(10207):1437-1449. doi: 10.1016/S0140-6736(19)31793-3. Epub 2019 Sep 12. Lancet. 2019. PMID: 31522849 Free PMC article. Clinical Trial.
-
A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow's milk allergy.J Allergy Clin Immunol. 2016 Apr;137(4):1103-1110.e11. doi: 10.1016/j.jaci.2015.10.005. Epub 2015 Nov 12. J Allergy Clin Immunol. 2016. PMID: 26581915 Free PMC article. Clinical Trial.
-
Efficacy and safety of oral immunotherapy with AR101 in European children with a peanut allergy (ARTEMIS): a multicentre, double-blind, randomised, placebo-controlled phase 3 trial.Lancet Child Adolesc Health. 2020 Oct;4(10):728-739. doi: 10.1016/S2352-4642(20)30234-0. Epub 2020 Jul 20. Lancet Child Adolesc Health. 2020. PMID: 32702315 Clinical Trial.
-
Clinical aspects of oral immunotherapy for the treatment of allergies.Semin Immunol. 2017 Apr;30:45-51. doi: 10.1016/j.smim.2017.07.008. Epub 2017 Aug 2. Semin Immunol. 2017. PMID: 28780220 Review.
-
Anti-IgE therapy versus allergen-specific immunotherapy for food allergy: weighing the pros and cons.Front Immunol. 2025 Jul 22;16:1617153. doi: 10.3389/fimmu.2025.1617153. eCollection 2025. Front Immunol. 2025. PMID: 40766311 Free PMC article. Review.
Cited by
-
World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) Guideline update - XIV - Recommendations on CMA immunotherapy.World Allergy Organ J. 2022 Apr 23;15(4):100646. doi: 10.1016/j.waojou.2022.100646. eCollection 2022 Apr. World Allergy Organ J. 2022. PMID: 35539896 Free PMC article.
-
CD33 recruitment inhibits IgE-mediated anaphylaxis and desensitizes mast cells to allergen.J Clin Invest. 2019 Mar 1;129(3):1387-1401. doi: 10.1172/JCI125456. Epub 2019 Feb 18. J Clin Invest. 2019. PMID: 30645205 Free PMC article.
-
Oral Immunotherapy in Children: Clinical Considerations and Practical Management.J Asthma Allergy. 2021 Dec 14;14:1497-1510. doi: 10.2147/JAA.S282696. eCollection 2021. J Asthma Allergy. 2021. PMID: 34934327 Free PMC article. Review.
-
Severe exercise-induced anaphylaxis in a hot and humid area successfully treated with omalizumab: a case report.Front Allergy. 2023 Jul 27;4:1228495. doi: 10.3389/falgy.2023.1228495. eCollection 2023. Front Allergy. 2023. PMID: 37577331 Free PMC article.
-
Advances and potential of omics studies for understanding the development of food allergy.Front Allergy. 2023 Mar 24;4:1149008. doi: 10.3389/falgy.2023.1149008. eCollection 2023. Front Allergy. 2023. PMID: 37034151 Free PMC article. Review.
References
-
- Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011; 128(1): e9–17. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

