Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials
- PMID: 29242041
- PMCID: PMC5757427
- DOI: 10.1016/S1470-2045(17)30777-5
Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials
Abstract
Background: Neoadjuvant chemotherapy (NACT) for early breast cancer can make breast-conserving surgery more feasible and might be more likely to eradicate micrometastatic disease than might the same chemotherapy given after surgery. We investigated the long-term benefits and risks of NACT and the influence of tumour characteristics on outcome with a collaborative meta-analysis of individual patient data from relevant randomised trials.
Methods: We obtained information about prerandomisation tumour characteristics, clinical tumour response, surgery, recurrence, and mortality for 4756 women in ten randomised trials in early breast cancer that began before 2005 and compared NACT with the same chemotherapy given postoperatively. Primary outcomes were tumour response, extent of local therapy, local and distant recurrence, breast cancer death, and overall mortality. Analyses by intention-to-treat used standard regression (for response and frequency of breast-conserving therapy) and log-rank methods (for recurrence and mortality).
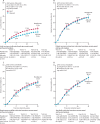
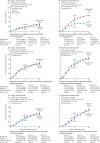
Findings: Patients entered the trials from 1983 to 2002 and median follow-up was 9 years (IQR 5-14), with the last follow-up in 2013. Most chemotherapy was anthracycline based (3838 [81%] of 4756 women). More than two thirds (1349 [69%] of 1947) of women allocated NACT had a complete or partial clinical response. Patients allocated NACT had an increased frequency of breast-conserving therapy (1504 [65%] of 2320 treated with NACT vs 1135 [49%] of 2318 treated with adjuvant chemotherapy). NACT was associated with more frequent local recurrence than was adjuvant chemotherapy: the 15 year local recurrence was 21·4% for NACT versus 15·9% for adjuvant chemotherapy (5·5% increase [95% CI 2·4-8·6]; rate ratio 1·37 [95% CI 1·17-1·61]; p=0·0001). No significant difference between NACT and adjuvant chemotherapy was noted for distant recurrence (15 year risk 38·2% for NACT vs 38·0% for adjuvant chemotherapy; rate ratio 1·02 [95% CI 0·92-1·14]; p=0·66), breast cancer mortality (34·4% vs 33·7%; 1·06 [0·95-1·18]; p=0·31), or death from any cause (40·9% vs 41·2%; 1·04 [0·94-1·15]; p=0·45).
Interpretation: Tumours downsized by NACT might have higher local recurrence after breast-conserving therapy than might tumours of the same dimensions in women who have not received NACT. Strategies to mitigate the increased local recurrence after breast-conserving therapy in tumours downsized by NACT should be considered-eg, careful tumour localisation, detailed pathological assessment, and appropriate radiotherapy.
Funding: Cancer Research UK, British Heart Foundation, UK Medical Research Council, and UK Department of Health.
Copyright © 2018 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures




Comment in
-
Neoadjuvant chemotherapy in breast cancer: more than just downsizing.Lancet Oncol. 2018 Jan;19(1):2-3. doi: 10.1016/S1470-2045(17)30914-2. Epub 2017 Dec 11. Lancet Oncol. 2018. PMID: 29242042 No abstract available.
-
Neoadjuvant chemotherapy for early breast cancer.Lancet Oncol. 2018 Mar;19(3):e128. doi: 10.1016/S1470-2045(18)30115-3. Lancet Oncol. 2018. PMID: 29508753 No abstract available.
-
Neoadjuvant chemotherapy for early breast cancer.Lancet Oncol. 2018 Mar;19(3):e129. doi: 10.1016/S1470-2045(18)30118-9. Lancet Oncol. 2018. PMID: 29508754 No abstract available.
-
Neoadjuvant chemotherapy for early breast cancer - Author's reply.Lancet Oncol. 2018 Mar;19(3):e130. doi: 10.1016/S1470-2045(18)30120-7. Lancet Oncol. 2018. PMID: 29508755 No abstract available.
References
-
- Rubens RD, Sexton S, Tong D, Winter PJ, Knight RK, Hayward JL. Combined chemotherapy and radiotherapy for locally advanced breast cancer. Eur J Cancer. 1980;16:351–356. - PubMed
-
- Mougalian SS, Soulos PR, Killelea BK. Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer. 2015;121:2544–2552. - PubMed
-
- Clough KB, Acosta-Marín V, Nos C. Rates of neoadjuvant chemotherapy and oncoplastic surgery for breast cancer surgery: a French national survey. Ann Surg Oncol. 2015;11:3504–3511. - PubMed
-
- Vugts G, Maaskant-Braat AJ, Nieuwenhuijzen GA, Roumen RM, Luiten EJ, Voogd AC. Patterns of care in the administration of neoadjuvant chemotherapy for breast cancer. A population-based study. Breast J. 2016;22:316–321. - PubMed
-
- Fisher B, Gunduz N, Coyle J, Rudock C, Saffer E. Presence of a growth-stimulating factor in serum following primary tumor removal in mice. Cancer Res. 1989;49:1996–2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

