Comparative Oligo-FISH Mapping: An Efficient and Powerful Methodology To Reveal Karyotypic and Chromosomal Evolution
- PMID: 29242292
- PMCID: PMC5788518
- DOI: 10.1534/genetics.117.300344
Comparative Oligo-FISH Mapping: An Efficient and Powerful Methodology To Reveal Karyotypic and Chromosomal Evolution
Abstract
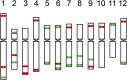
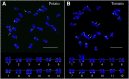
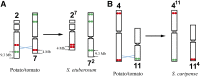
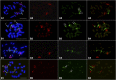
Developing the karyotype of a eukaryotic species relies on identification of individual chromosomes, which has been a major challenge for most nonmodel plant and animal species. We developed a novel chromosome identification system by selecting and labeling oligonucleotides (oligos) located in specific regions on every chromosome. We selected a set of 54,672 oligos (45 nt) based on single copy DNA sequences in the potato genome. These oligos generated 26 distinct FISH signals that can be used as a "bar code" or "banding pattern" to uniquely label each of the 12 chromosomes from both diploid and polyploid (4× and 6×) potato species. Remarkably, the same bar code can be used to identify the 12 homeologous chromosomes among distantly related Solanum species, including tomato and eggplant. Accurate karyotypes based on individually identified chromosomes were established in six Solanum species that have diverged for >15 MY. These six species have maintained a similar karyotype; however, modifications to the FISH signal bar code led to the discovery of two reciprocal chromosomal translocations in Solanum etuberosum and S. caripense We also validated these translocations by oligo-based chromosome painting. We demonstrate that the oligo-based FISH techniques are powerful new tools for chromosome identification and karyotyping research, especially for nonmodel plant species.
Keywords: chromosome identification; chromosome painting; karyotype; oligo-FISH; translocation.
Copyright © 2018 by the Genetics Society of America.
Figures


References
-
- Anderson L. K., Stack S. M., Mitchell J. B., 1982. An investigation of the basis of a current hypothesis for the lack of G-banding in plant chromosomes. Exp. Cell Res. 138: 433–436. - PubMed
-
- Badaeva E. D., Jiang J. M., Gill B. S., 1995. Detection of intergenomic translocations with centromeric and noncentromeric breakpoints in Triticum araraticum - mechanism of origin and adaptive significance. Genome 38: 976–981. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

