DNA damage causes rapid accumulation of phosphoinositides for ATR signaling
- PMID: 29242514
- PMCID: PMC5730617
- DOI: 10.1038/s41467-017-01805-9
DNA damage causes rapid accumulation of phosphoinositides for ATR signaling
Abstract
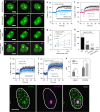
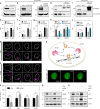
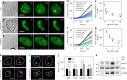
Phosphoinositide lipids (PPIs) are enriched in the nucleus and are accumulated at DNA damage sites. Here, we investigate roles of nuclear PPIs in DNA damage response by sequestering specific PPIs with the expression of nuclear-targeted PH domains, which inhibits recruitment of Ataxia telangiectasia and Rad3-related protein (ATR) and reduces activation of Chk1. PPI-binding domains rapidly (< 1 s) accumulate at damage sites with local enrichment of PPIs. Accumulation of PIP3 in complex with the nuclear receptor protein, SF1, at damage sites requires phosphorylation by inositol polyphosphate multikinase (IPMK) and promotes nuclear actin assembly that is required for ATR recruitment. Suppressed ATR recruitment/activation is confirmed with latrunculin A and wortmannin treatment as well as IPMK or SF1 depletion. Other DNA repair pathways involving ATM and DNA-PKcs are unaffected by PPI sequestration. Together, these findings reveal that nuclear PPI metabolism mediates an early damage response through the IPMK-dependent pathway to specifically recruit ATR.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
Simulated Microgravity Promotes Cell Apoptosis Through Suppressing Uev1A/TICAM/TRAF/NF-κB-Regulated Anti-Apoptosis and p53/PCNA- and ATM/ATR-Chk1/2-Controlled DNA-Damage Response Pathways.J Cell Biochem. 2016 Sep;117(9):2138-48. doi: 10.1002/jcb.25520. Epub 2016 Mar 8. J Cell Biochem. 2016. PMID: 26887372
-
Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage.Nature. 2005 Mar 31;434(7033):605-11. doi: 10.1038/nature03442. Epub 2005 Mar 2. Nature. 2005. PMID: 15758953
-
ATR/Chk1 signaling induces autophagy through sumoylated RhoB-mediated lysosomal translocation of TSC2 after DNA damage.Nat Commun. 2018 Oct 8;9(1):4139. doi: 10.1038/s41467-018-06556-9. Nat Commun. 2018. PMID: 30297842 Free PMC article.
-
The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer.Adv Cancer Res. 2010;108:73-112. doi: 10.1016/B978-0-12-380888-2.00003-0. Adv Cancer Res. 2010. PMID: 21034966 Review.
-
Ataxia telangiectasia and Rad3-related inhibitors and cancer therapy: where we stand.J Hematol Oncol. 2019 Apr 24;12(1):43. doi: 10.1186/s13045-019-0733-6. J Hematol Oncol. 2019. PMID: 31018854 Free PMC article. Review.
Cited by
-
Nuclear F-actin assembly on damaged chromatin is regulated by DYRK1A and Spir1 phosphorylation.Nucleic Acids Res. 2024 Aug 27;52(15):8897-8912. doi: 10.1093/nar/gkae574. Nucleic Acids Res. 2024. PMID: 38966995 Free PMC article.
-
Nuclear Actin: From Discovery to Function.Anat Rec (Hoboken). 2018 Dec;301(12):1999-2013. doi: 10.1002/ar.23959. Epub 2018 Nov 1. Anat Rec (Hoboken). 2018. PMID: 30312531 Free PMC article. Review.
-
A sterol-PI(4)P exchanger modulates the Tel1/ATM axis of the DNA damage response.EMBO J. 2023 Aug 1;42(15):e112684. doi: 10.15252/embj.2022112684. Epub 2023 Jun 12. EMBO J. 2023. PMID: 37303233 Free PMC article.
-
Nuclear actin polymerization rapidly mediates replication fork remodeling upon stress by limiting PrimPol activity.Nat Commun. 2023 Nov 28;14(1):7819. doi: 10.1038/s41467-023-43183-5. Nat Commun. 2023. PMID: 38016948 Free PMC article.
-
Increased H3K9me3 and F-Actin Reorganization in the Rapid Adaptive Response to Hypergravity in Human T Lymphocytes.Int J Mol Sci. 2023 Dec 7;24(24):17232. doi: 10.3390/ijms242417232. Int J Mol Sci. 2023. PMID: 38139061 Free PMC article.
References
-
- Dieck CB, Wood A, Brglez I, Rojas-Pierce M, Boss WF. Increasing phosphatidylinositol (4,5) bisphosphate biosynthesis affects plant nuclear lipids and nuclear functions. Plant physiology and biochemistry: PPB / Societe francaise de physiologie vegetale. 2012;57:32–44. doi: 10.1016/j.plaphy.2012.05.011. - DOI - PMC - PubMed
-
- Smith CD, Wells WW. Phosphorylation of rat liver nuclear envelopes. II. Characterization of in vitro lipid phosphorylation. J. Biol. Chem. 1983;258:9368–9373. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

