Inhibition of human kynurenine aminotransferase isozymes by estrogen and its derivatives
- PMID: 29242525
- PMCID: PMC5730616
- DOI: 10.1038/s41598-017-17979-7
Inhibition of human kynurenine aminotransferase isozymes by estrogen and its derivatives
Abstract
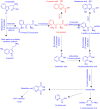
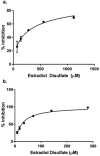
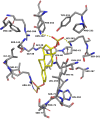
The kynurenine aminotransferase (KAT) enzymes are pyridoxal 5'-phosphate-dependent homodimers that catalyse the irreversible transamination of kynurenine into kynurenic acid (KYNA) in the tryptophan metabolic pathway. Kynurenic acid is implicated in cognitive diseases such as schizophrenia, and several inhibitors have been reported that selectively target KAT-II as it is primarily responsible for kynurenic acid production in the human brain. Not only is schizophrenia a sexually dimorphic condition, but women that have schizophrenia have reduced estrogen levels in their serum. Estrogens are also known to interact in the kynurenine pathway therefore exploring these interactions can yield a better understanding of the condition and improve approaches in ameliorating its effects. Enzyme inhibitory assays and binding studies showed that estradiol disulfate is a strong inhibitor of KAT-I and KAT-II (IC50: 291.5 μM and 26.3 μM, respectively), with estradiol, estradiol 3-sulfate and estrone sulfate being much weaker (IC50 > 2 mM). Therefore it is possible that estrogen levels can dictate the balance of kynurenic acid in the brain. Inhibition assay results and modelling suggests that the 17-sulfate moiety in estradiol disulfate is very important in improving its potency as an inhibitor, increasing the inhibition by approximately 10-100 fold compared to estradiol.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Improvement of kynurenine aminotransferase-II inhibitors guided by mimicking sulfate esters.PLoS One. 2018 Apr 24;13(4):e0196404. doi: 10.1371/journal.pone.0196404. eCollection 2018. PLoS One. 2018. PMID: 29689093 Free PMC article.
-
Kynurenine Aminotransferase Isozyme Inhibitors: A Review.Int J Mol Sci. 2016 Jun 15;17(6):946. doi: 10.3390/ijms17060946. Int J Mol Sci. 2016. PMID: 27314340 Free PMC article. Review.
-
Fragment Screening of Human Kynurenine Aminotransferase-II.SLAS Discov. 2018 Jul;23(6):511-519. doi: 10.1177/2472555218764620. Epub 2018 Mar 14. SLAS Discov. 2018. PMID: 29537924
-
Quantum mechanics/molecular mechanics (QM/MM) modeling of the irreversible transamination of L-kynurenine to kynurenic acid: the round dance of kynurenine aminotransferase II.Biochim Biophys Acta. 2009 Dec;1794(12):1802-12. doi: 10.1016/j.bbapap.2009.08.016. Epub 2009 Aug 26. Biochim Biophys Acta. 2009. PMID: 19715778
-
Kynurenine Aminotransferases and the Prospects of Inhibitors for the Treatment of Schizophrenia.Curr Med Chem. 2015;22(24):2902-18. doi: 10.2174/0929867322666150608094054. Curr Med Chem. 2015. PMID: 26051411 Review.
Cited by
-
Effect of hormone therapy on tryptophan metabolism and atherosclerosis among postmenopausal women.Climacteric. 2025 Jun 13:1-7. doi: 10.1080/13697137.2025.2509838. Online ahead of print. Climacteric. 2025. PMID: 40512395
-
Exercised accelerated the production of muscle-derived kynurenic acid in skeletal muscle and alleviated the postmenopausal osteoporosis through the Gpr35/NFκB p65 pathway.J Orthop Translat. 2022 Apr 4;35:1-12. doi: 10.1016/j.jot.2022.03.003. eCollection 2022 Jul. J Orthop Translat. 2022. PMID: 35846727 Free PMC article.
-
A Potential Interface between the Kynurenine Pathway and Autonomic Imbalance in Schizophrenia.Int J Mol Sci. 2021 Sep 16;22(18):10016. doi: 10.3390/ijms221810016. Int J Mol Sci. 2021. PMID: 34576179 Free PMC article. Review.
-
Associations between estrogen and progesterone, the kynurenine pathway, and inflammation in the post-partum.J Affect Disord. 2021 Feb 15;281:9-12. doi: 10.1016/j.jad.2020.10.052. Epub 2020 Oct 29. J Affect Disord. 2021. PMID: 33278766 Free PMC article.
-
Tryptophan Metabolism: A Versatile Area Providing Multiple Targets for Pharmacological Intervention.Egypt J Basic Clin Pharmacol. 2019;9:10.32527/2019/101415. doi: 10.32527/2019/101415. Egypt J Basic Clin Pharmacol. 2019. PMID: 31105983 Free PMC article.
References
-
- Picchioni MM, Murray RM. Schizophrenia. BMJ. 2007;335:91–95. doi: 10.1136/bmj.39227.616447.BE. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

