Nuclear lamin A/C harnesses the perinuclear apical actin cables to protect nuclear morphology
- PMID: 29242553
- PMCID: PMC5730574
- DOI: 10.1038/s41467-017-02217-5
Nuclear lamin A/C harnesses the perinuclear apical actin cables to protect nuclear morphology
Erratum in
-
Author Correction: Nuclear lamin A/C harnesses the perinuclear apical actin cables to protect nuclear morphology.Nat Commun. 2018 Mar 13;9(1):1115. doi: 10.1038/s41467-018-03450-2. Nat Commun. 2018. PMID: 29535303 Free PMC article.
Abstract
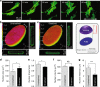
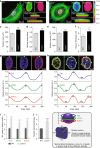
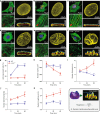
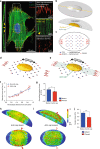
The distinct spatial architecture of the apical actin cables (or actin cap) facilitates rapid biophysical signaling between extracellular mechanical stimuli and intracellular responses, including nuclear shaping, cytoskeletal remodeling, and the mechanotransduction of external forces into biochemical signals. These functions are abrogated in lamin A/C-deficient mouse embryonic fibroblasts that recapitulate the defective nuclear organization of laminopathies, featuring disruption of the actin cap. However, how nuclear lamin A/C mediates the ability of the actin cap to regulate nuclear morphology remains unclear. Here, we show that lamin A/C expressing cells can form an actin cap to resist nuclear deformation in response to physiological mechanical stresses. This study reveals how the nuclear lamin A/C-mediated formation of the perinuclear apical actin cables protects the nuclear structural integrity from extracellular physical disturbances. Our findings highlight the role of the physical interactions between the cytoskeletal network and the nucleus in cellular mechanical homeostasis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

