Rheological Droplet Interface Bilayers (rheo-DIBs): Probing the Unstirred Water Layer Effect on Membrane Permeability via Spinning Disk Induced Shear Stress
- PMID: 29242597
- PMCID: PMC5730560
- DOI: 10.1038/s41598-017-17883-0
Rheological Droplet Interface Bilayers (rheo-DIBs): Probing the Unstirred Water Layer Effect on Membrane Permeability via Spinning Disk Induced Shear Stress
Abstract
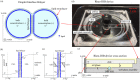
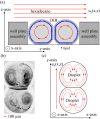
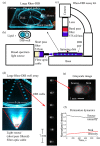
A new rheological droplet interface bilayer (rheo-DIB) device is presented as a tool to apply shear stress on biological lipid membranes. Despite their exciting potential for affecting high-throughput membrane translocation studies, permeability assays conducted using DIBs have neglected the effect of the unstirred water layer (UWL). However as demonstrated in this study, neglecting this phenomenon can cause significant underestimates in membrane permeability measurements which in turn limits their ability to predict key processes such as drug translocation rates across lipid membranes. With the use of the rheo-DIB chip, the effective bilayer permeability can be modulated by applying shear stress to the droplet interfaces, inducing flow parallel to the DIB membranes. By analysing the relation between the effective membrane permeability and the applied stress, both the intrinsic membrane permeability and UWL thickness can be determined for the first time using this model membrane approach, thereby unlocking the potential of DIBs for undertaking diffusion assays. The results are also validated with numerical simulations.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Barlow, N. E. et al. Multiplexed droplet Interface bilayer formation. Lab on a chip (2016). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

