Ubiquitin ligases in oncogenic transformation and cancer therapy
- PMID: 29242641
- PMCID: PMC6054770
- DOI: 10.1038/nrc.2017.105
Ubiquitin ligases in oncogenic transformation and cancer therapy
Abstract
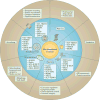
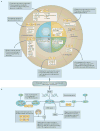
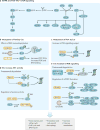
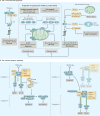
The cellular response to external stress signals and DNA damage depends on the activity of ubiquitin ligases (E3s), which regulate numerous cellular processes, including homeostasis, metabolism and cell cycle progression. E3s recognize, interact with and ubiquitylate protein substrates in a temporally and spatially regulated manner. The topology of the ubiquitin chains dictates the fate of the substrates, marking them for recognition and degradation by the proteasome or altering their subcellular localization or assembly into functional complexes. Both genetic and epigenetic alterations account for the deregulation of E3s in cancer. Consequently, the stability and/or activity of E3 substrates are also altered, in some cases leading to downregulation of tumour-suppressor activities and upregulation of oncogenic activities. A better understanding of the mechanisms underlying E3 regulation and function in tumorigenesis is expected to identify novel prognostic markers and to enable the development of the next generation of anticancer therapies. This Review summarizes the oncogenic and tumour-suppressor roles of selected E3s and highlights novel opportunities for therapeutic intervention.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Ciechanover A. The unravelling of the ubiquitin system. Nat. Rev. Mol. Cell Biol. 2015;16:322–324. This is a timely updated essay on the fundamentals of the ubiquitin system. - PubMed
-
- Foot N, Henshall T, Kumar S. Ubiquitination and the regulation of membrane proteins. Physiol. Rev. 2017;97:253–281. - PubMed
-
- Khaminets A, Behl C, Dikic I. Ubiquitin-dependent and independent signals in selective autophagy. Trends Cell Biol. 2016;26:6–16. This is a detailed review on the regulation of autophagy by ubiquitin-related and non-related pathways. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

