Highly lipophilic fluorescent dyes in nano-emulsions: towards bright non-leaking nano-droplets
- PMID: 29242742
- PMCID: PMC5726488
- DOI: 10.1039/C2RA21544F
Highly lipophilic fluorescent dyes in nano-emulsions: towards bright non-leaking nano-droplets
Abstract
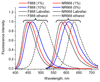
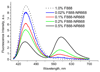
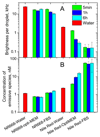
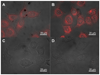
Dye-loaded lipid nano-droplets present an attractive alternative to inorganic nanoparticles, as they are composed of non-toxic biodegradable materials and easy to prepare. However, to achieve high fluorescence brightness, the nano-droplets have to be heavily loaded with the dyes avoiding fluorescence self-quenching and release (leakage) of the encapsulated dyes from the nano-droplets in biological media. In the present work, we have designed highly lipophilic fluorescent derivatives of 3-alkoxyflavone (F888) and Nile Red (NR668) that can be encapsulated in the lipophilic core of stable nano-emulsion droplets at exceptionally high concentrations in the oil core, i.e. up to 170 mM and 17 mM, respectively, corresponding to ~ 830 and 80 dyes per 40-nm droplet. Despite this high loading, these dyes keep high fluorescence quantum yield and thus, provide high nano-droplet brightness, probably due to their bulky structure preventing self-quenching. Moreover, simultaneous encapsulation of both dyes at high concentrations in single nano-droplets allows observation of FRET. FRET and fluorescence correlation spectroscopy (FCS) studies showed that NR668 release in the serum-containing medium is very slow, while the reference hydrophobic dye Nile Red leaks immediately. This drastic difference in the leakage profile between NR668 and Nile Red was confirmed by in vitro cellular studies as well as by in vivo angiography imaging on zebrafish model, where the NR668-loaded nano-droplets remained in the blood circulation, while the parent Nile Red leaked rapidly from the droplets distributing all over the animal body. This study suggests new molecular design strategies for obtaining bright nano-droplets without dye leakage and their use as efficient and stable optical contrast agents in vitro and in vivo.
Keywords: Förster resonance energy transfer; dye release; fluorescence correlation spectroscopy; fluorescent lipid nano-droplets; intra-vital imaging; nano-emulsions.
Figures



References
-
- Brigger I, Dubernet C, Couvreur P. Adv Drug Deliv Rev. 2002;54:631. - PubMed
-
- Horn D, Rieger J. Angew Chem Int Ed. 2001;40:4330. - PubMed
-
- Sharma P, Brown S, Walter G, Santra S, Moudgil B. Adv Colloid Interface Sci. 2006;123-126:471. - PubMed
-
- Lu J, Liong M, Zink JI, Tamanoi F. Small. 2007;3:1341. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
