ESTABLISHING A LIVE CARTILAGE-ON-CARTILAGE INTERFACE FOR TRIBOLOGICAL TESTING
- PMID: 29242820
- PMCID: PMC5726278
- DOI: 10.1016/j.biotri.2016.11.002
ESTABLISHING A LIVE CARTILAGE-ON-CARTILAGE INTERFACE FOR TRIBOLOGICAL TESTING
Abstract
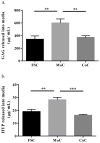
Mechano-biochemical wear encompasses the tribological interplay between biological and mechanical mechanisms responsible for cartilage wear and degradation. The aim of this study was to develop and start validating a novel tribological testing system, which better resembles the natural joint environment through incorporating a live cartilage-on-cartilage articulating interface, joint specific kinematics, and the application of controlled mechanical stimuli for the measurement of biological responses in order to study the mechano-biochemical wear of cartilage. The study entailed two parts. In Part 1, the novel testing rig was used to compare two bearing systems: (a) cartilage articulating against cartilage (CoC) and (b) metal articulating against cartilage (MoC). The clinically relevant MoC, which is also a common tribological interface for evaluating cartilage wear, should produce more wear to agree with clinical observations. In Part II, the novel testing system was used to determine how wear is affected by tissue viability in live and dead CoC articulations. For both parts, bovine cartilage explants were harvested and tribologically tested for three consecutive days. Wear was defined as release of glycosaminoglycans into the media and as evaluation of the tissue structure. For Part I, we found that the live CoC articulation did not cause damage to the cartilage, to the extent of being comparable to the free swelling controls, whereas the MoC articulation caused decreased cell viability, extracellular matrix disruption, and increased wear when compared to CoC, and consistent with clinical data. These results provided confidence that this novel testing system will be adequate to screen new biomaterials for articulation against cartilage, such as in hemiarthroplasty. For Part II, the live and dead cartilage articulation yielded similar wear as determined by the release of proteoglycans and aggrecan fragments, suggesting that keeping the cartilage alive may not be essential for short term wear tests. However, the biosynthesis of glycosaminoglycans was significantly higher due to live CoC articulation than due to the corresponding live free swelling controls, indicating that articulation stimulated cell activity. Moving forward, the cell response to mechanical stimuli and the underlying mechano-biochemical wear mechanisms need to be further studied for a complete picture of tissue degradation.
Keywords: cartilage; cartilage mechanics; joint motion simulation; tribology- wear.
Conflict of interest statement
Conflicts of Interest: None
Figures






References
-
- Rabinowicz E. Friction and Wear of Materials. 2. Wiley-Interscience; New York, NY: 1995.
-
- Waldman SD, Couto DC, Grynpas MD, Pilliar RM, Kandel RA. Multi-axial mechanical stimulation of tissue engineered cartilage: review. Eur Cell Mater. 2007;13:66–73. discussion 73–74. - PubMed
-
- Mankin H, Mow VC, Buckwalter JA, Iannotti J, Ratcliffe A. Orthop Basic Sci. Chapter 1. American Academy of Orthopaedic Surgeons; Columbus, OH: 1994. Form and function of articular cartilage; pp. 1–44.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
