Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: a multicenter, randomized controlled trial
- PMID: 29243164
- PMCID: PMC5823751
- DOI: 10.1007/s00520-017-4013-0
Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: a multicenter, randomized controlled trial
Abstract
Purpose: Over half of all cancer patients receiving taxane-, platinum-, or vinca alkaloid-based chemotherapy experience chemotherapy-induced peripheral neuropathy (CIPN), which includes numbness, tingling, pain, cold sensitivity, and motor impairment in the hands and feet. CIPN is a dose-limiting toxicity, potentially increasing mortality. There are no FDA-approved drugs to treat CIPN, and behavioral interventions such as exercise are promising yet understudied. This secondary analysis of our nationwide phase III randomized controlled trial of exercise for fatigue examines (1) effects of exercise on CIPN symptoms, (2) factors that predict CIPN symptoms, and (3) factors that moderate effects of exercise on CIPN symptoms.
Methods: Cancer patients (N = 355, 56 ± 11 years, 93% female, 79% breast cancer) receiving taxane-, platinum-, or vinca alkaloid-based chemotherapy were randomized to chemotherapy or chemotherapy plus Exercise for Cancer Patients (EXCAP©®). EXCAP is a standardized, individualized, moderate-intensity, home-based, six-week progressive walking and resistance exercise program. Patients reported CIPN symptoms of numbness and tingling and hot/coldness in hands/feet (0-10 scales) pre- and post-intervention. We explored baseline neuropathy, sex, age, body mass index, cancer stage, and cancer type as possible factors associated with CIPN symptoms and exercise effectiveness.
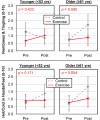
Results: Exercise reduced CIPN symptoms of hot/coldness in hands/feet (-0.46 units, p = 0.045) and numbness and tingling (- 0.42 units, p = 0.061) compared to the control. Exercise reduced CIPN symptoms more for patients who were older (p = 0.086), male (p = 0.028), or had breast cancer (p = 0.076).
Conclusions: Exercise appears to reduce CIPN symptoms in patients receiving taxane-, platinum-, or vinca alkaloid-based chemotherapy. Clinicians should consider prescribing exercise for these patients.
Trial registration: Clinical Trials.gov , # NCT00924651, http://www.clinicaltrials.gov .
Keywords: CIPN; Exercise; Neuropathy.
Conflict of interest statement
Figures


Comment in
-
Physical interventions for patients suffering from chemotherapy-induced polyneuropathy.Support Care Cancer. 2018 Apr;26(4):1017-1018. doi: 10.1007/s00520-018-4071-y. Epub 2018 Jan 27. Support Care Cancer. 2018. PMID: 29374785 No abstract available.
-
Letter to the editor-comments on Kleckner et al. (2018) "Effects of exercise during chemotherapy-induced peripheral neuropathy: a multicenter, randomized controlled trial".Support Care Cancer. 2019 Jan;27(1):3-4. doi: 10.1007/s00520-018-4361-4. Epub 2018 Jul 22. Support Care Cancer. 2019. PMID: 30032400 No abstract available.
-
Chemotherapy-induced peripheral neuropathy-more high-quality research is needed.Support Care Cancer. 2019 Jan;27(1):5-6. doi: 10.1007/s00520-018-4371-2. Epub 2018 Jul 25. Support Care Cancer. 2019. PMID: 30046880 No abstract available.
References
-
- Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014;155(12):2461–70. - PubMed
-
- Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, Hoang-Xuan K, Lanteri-Minet M, Grant R, Huddart R, Moynihan C, Maher J, Lucey R, E.Q.o.L. Group The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer. 2005;41(8):1135–9. - PubMed
-
- Lyman GH. Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Canc Netw. 2009;7(1):99–108. - PubMed
-
- Beijers A, Mols F, Dercksen W, Driessen C, Vreugdenhil G. Chemotherapy-induced peripheral neuropathy and impact on quality of life 6 months after treatment with chemotherapy. J Community Support Oncol. 2014;12(11):401–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

