Diverse functions of homologous actin isoforms are defined by their nucleotide, rather than their amino acid sequence
- PMID: 29244021
- PMCID: PMC5794254
- DOI: 10.7554/eLife.31661
Diverse functions of homologous actin isoforms are defined by their nucleotide, rather than their amino acid sequence
Abstract
β- and γ-cytoplasmic actin are nearly indistinguishable in their amino acid sequence, but are encoded by different genes that play non-redundant biological roles. The key determinants that drive their functional distinction are unknown. Here, we tested the hypothesis that β- and γ-actin functions are defined by their nucleotide, rather than their amino acid sequence, using targeted editing of the mouse genome. Although previous studies have shown that disruption of β-actin gene critically impacts cell migration and mouse embryogenesis, we demonstrate here that generation of a mouse lacking β-actin protein by editing β-actin gene to encode γ-actin protein, and vice versa, does not affect cell migration and/or organism survival. Our data suggest that the essential in vivo function of β-actin is provided by the gene sequence independent of the encoded protein isoform. We propose that this regulation constitutes a global 'silent code' mechanism that controls the functional diversity of protein isoforms.
Keywords: actin; biochemistry; cell biology; coding sequence; isoforms; mouse.
Conflict of interest statement
PV, SK, NL, YW, SS, JW, SS, DD, AK No competing interests declared
Figures
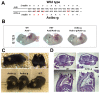

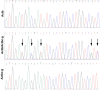
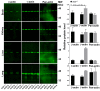

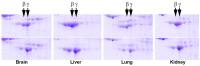
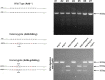


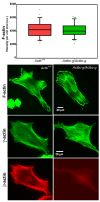
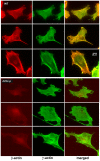

Comment in
-
Fundamental differences.Elife. 2018 Feb 1;7:e34477. doi: 10.7554/eLife.34477. Elife. 2018. PMID: 29388551 Free PMC article.
References
-
- Burgess-Cassler A, Johansen JJ, Santek DA, Ide JR, Kendrick NC. Computerized quantitative analysis of coomassie-blue-stained serum proteins separated by two-dimensional electrophoresis. Clinical Chemistry. 1989;35:2297–2304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

