β-Cell Control of Insulin Production During Starvation-Refeeding in Male Rats
- PMID: 29244064
- PMCID: PMC5776497
- DOI: 10.1210/en.2017-03120
β-Cell Control of Insulin Production During Starvation-Refeeding in Male Rats
Abstract
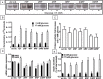
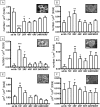
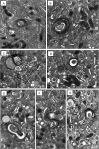
Mammalian metabolism has evolved to adapt to changes in nutrient status. Insulin, the key anabolic hormone, facilitates intracellular storage of nutrient fuels and plays a pivotal role in the transition away from catabolism upon refeeding. Although circulating insulin relative to nutrient levels has been well characterized during fasting and refeeding, how pancreatic β-cell biology caters to acute changes in insulin demand has not been sufficiently addressed. Here, we examined the dynamics of (pro)insulin production and associated changes in β-cell ultrastructure during refeeding after a 72-hour fast in male rats. We found that fasted β-cells had marked degranulation, which inversely coordinated with the upregulation of autophagolysomal and lysosomal organelles. There was also expanded Golgi that correlated with enhanced (pro)insulin biosynthetic capacity but, conversely, blunted in vivo insulin secretion. Within 4 to 6 hours of refeeding, proinsulin biosynthesis, cellular ultrastructure, in vivo insulin secretion, and glucose tolerance normalized to levels near those of fed control animals, indicating a rapid replenishment of normal insulin secretory capacity. Thus, during a prolonged fast, the β-cell protects against hypoglycemia by markedly reducing insulin secretory capacity in vivo but is simultaneously poised to efficiently increase (pro)insulin production upon refeeding to effectively return normal insulin secretory capacity within hours.
Copyright © 2018 Endocrine Society.
Figures



References
-
- Chakravarthy MV, Booth FW. Eating, exercise, and “thrifty” genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J Appl Physiol (1985). 2004;96(1):3–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

