Origins of Enzyme Catalysis: Experimental Findings for C-H Activation, New Models, and Their Relevance to Prevailing Theoretical Constructs
- PMID: 29244501
- PMCID: PMC5812730
- DOI: 10.1021/jacs.7b08418
Origins of Enzyme Catalysis: Experimental Findings for C-H Activation, New Models, and Their Relevance to Prevailing Theoretical Constructs
Abstract
The physical basis for enzymatic rate accelerations is a subject of great fundamental interest and of direct relevance to areas that include the de novo design of green catalysts and the pursuit of new drug regimens. Extensive investigations of C-H activating systems have provided considerable insight into the relationship between an enzyme's overall structure and the catalytic chemistry at its active site. This Perspective highlights recent experimental data for two members of distinct, yet iconic C-H activation enzyme classes, lipoxygenases and prokaryotic alcohol dehydrogenases. The data necessitate a reformulation of the dominant textbook definition of biological catalysis. A multidimensional model emerges that incorporates a range of protein motions that can be parsed into a combination of global stochastic conformational thermal fluctuations and local donor-acceptor distance sampling. These motions are needed to achieve a high degree of precision with regard to internuclear distances, geometries, and charges within the active site. The available model also suggests a physical framework for understanding the empirical enthalpic barrier in enzyme-catalyzed processes. We conclude by addressing the often conflicting interface between computational and experimental chemists, emphasizing the need for computation to predict experimental results in advance of their measurement.
Conflict of interest statement
Notes: The authors declare no competing financial interest.
Figures

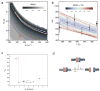
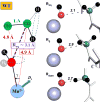
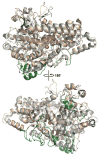
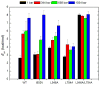


Similar articles
-
Picosecond-resolved fluorescent probes at functionally distinct tryptophans within a thermophilic alcohol dehydrogenase: relationship of temperature-dependent changes in fluorescence to catalysis.J Phys Chem B. 2014 Jun 12;118(23):6049-61. doi: 10.1021/jp500825x. Epub 2014 Jun 3. J Phys Chem B. 2014. PMID: 24892947 Free PMC article.
-
Role of dynamics in enzyme catalysis: substantial versus semantic controversies.Acc Chem Res. 2015 Feb 17;48(2):466-73. doi: 10.1021/ar500322s. Epub 2014 Dec 24. Acc Chem Res. 2015. PMID: 25539442 Free PMC article. Review.
-
A model reaction assesses contribution of H-tunneling and coupled motions to enzyme catalysis.J Org Chem. 2012 Aug 17;77(16):6825-33. doi: 10.1021/jo300879r. Epub 2012 Aug 9. J Org Chem. 2012. PMID: 22834675
-
Identification of a long-range protein network that modulates active site dynamics in extremophilic alcohol dehydrogenases.J Biol Chem. 2013 May 17;288(20):14087-14097. doi: 10.1074/jbc.M113.453951. Epub 2013 Mar 22. J Biol Chem. 2013. PMID: 23525111 Free PMC article.
-
Dynamically achieved active site precision in enzyme catalysis.Acc Chem Res. 2015 Feb 17;48(2):449-56. doi: 10.1021/ar5003347. Epub 2014 Dec 24. Acc Chem Res. 2015. PMID: 25539048 Free PMC article. Review.
Cited by
-
Structural and Functional Biology of Mammalian ALOX Isoforms with Particular Emphasis on Enzyme Dimerization and Their Allosteric Properties.Int J Mol Sci. 2024 Nov 9;25(22):12058. doi: 10.3390/ijms252212058. Int J Mol Sci. 2024. PMID: 39596127 Free PMC article. Review.
-
Impact of Mutations on the Binding Pocket of Soybean Lipoxygenase: Implications for Proton-Coupled Electron Transfer.J Phys Chem Lett. 2018 Nov 15;9(22):6444-6449. doi: 10.1021/acs.jpclett.8b02945. Epub 2018 Oct 29. J Phys Chem Lett. 2018. PMID: 30359035 Free PMC article.
-
Theoretical Study of C-H Bond Cleavage via Concerted Proton-Coupled Electron Transfer in Fluorenyl-Benzoates.J Am Chem Soc. 2018 Nov 21;140(46):15641-15645. doi: 10.1021/jacs.8b10461. Epub 2018 Nov 8. J Am Chem Soc. 2018. PMID: 30383371 Free PMC article.
-
3D Variability Analysis Reveals a Hidden Conformational Change Controlling Ammonia Transport in Human Asparagine Synthetase.bioRxiv [Preprint]. 2024 Sep 5:2023.05.16.541009. doi: 10.1101/2023.05.16.541009. bioRxiv. 2024. Update in: Nat Commun. 2024 Dec 3;15(1):10538. doi: 10.1038/s41467-024-54912-9. PMID: 37292727 Free PMC article. Updated. Preprint.
-
Tailoring Proteins to Re-Evolve Nature: A Short Review.Mol Biotechnol. 2018 Dec;60(12):946-974. doi: 10.1007/s12033-018-0122-3. Mol Biotechnol. 2018. PMID: 30264233 Review.
References
-
- Jencks WP. Binding energy, specificity, and enzymic catalysis: the circe effect. 1975. - PubMed
-
- Zhang XY, Houk KN. Acc Chem Res. 2005;38:379–385. - PubMed
-
- Walsh C. Enzymatic reaction mechanisms. W. H. Freeman; San Francisco: 1979.
-
- Frey PA, Hegeman AD. Enzymatic reaction mechanisms. Oxford University Press; Oxford ; New York: 2007.
-
- De Clercq E. Rev Med Virol. 2009;19:287–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

