Dual modulation of human hepatic zonation via canonical and non-canonical Wnt pathways
- PMID: 29244788
- PMCID: PMC5750478
- DOI: 10.1038/emm.2017.226
Dual modulation of human hepatic zonation via canonical and non-canonical Wnt pathways
Abstract
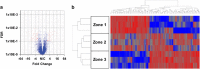
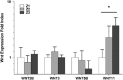
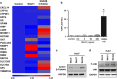
The hepatic lobule is divided into three zones along the portal-central vein axis. Hepatocytes within each zone exhibit a distinctive gene expression profile that coordinates their metabolic compartmentalization. The zone-dependent heterogeneity of hepatocytes has been hypothesized to result from the differential degree of exposure to oxygen, nutrition and gut-derived toxins. In addition, the gradient of Wnt signaling that increases towards the central vein seen in rodent models is believed to play a critical role in shaping zonation. Furthermore, hepatic zonation is coupled to the site of the homeostatic renewal of hepatocytes. Despite its critical role, the regulatory mechanisms that determine the distinctive features of zonation and its relevance to humans are not well understood. The present study first conducted a comprehensive zone-dependent transcriptome analysis of normal human liver using laser capture microdissection. Upstream pathway analysis revealed the signatures of host responses to gut-derived toxins in the periportal zone, while both the canonical Wnt pathway and the xenobiotic response pathway govern the perivenular zone. Furthermore, we found that the hypoxic environment of the perivenular zone promotes Wnt11 expression in hepatocytes, which then regulates unique gene expression via activation of the non-canonical Wnt pathway. In summary, our study reports the comprehensive zonation-dependent transcriptome of the normal human liver. Our analysis revealed that the LPS response pathway shapes the characteristics of periportal hepatocytes. By contrast, the perivenular zone is regulated by a combination of three distinct pathways: the xenobiotic response pathway, canonical Wnt signaling, and hypoxia-induced noncanonical Wnt signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Rappaport AM, Borowy ZJ, Lougheed WM, Lotto WN. Subdivision of hexagonal liver lobules into a structural and functional unit; role in hepatic physiology and pathology. Anat Rec 1954; 119: 11–33. - PubMed
-
- Jungermann K, Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology 2000; 31: 255–260. - PubMed
-
- Rappaport AM. The microcirculatory acinar concept of normal and pathological hepatic structure. Beitr Pathol 1976; 157: 215–243. - PubMed
-
- Torre C, Perret C, Colnot S. Molecular determinants of liver zonation. Prog Mol Biol Transl Sci 2010; 97: 127–150. - PubMed
-
- Jungermann K. Metabolic zonation of liver parenchyma. Semin Liver Dis 1988; 8: 329–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

