Impact of increased influenza vaccination in 2-3-year-old children on disease burden within the general population: A Bayesian model-based approach
- PMID: 29244811
- PMCID: PMC5731690
- DOI: 10.1371/journal.pone.0186739
Impact of increased influenza vaccination in 2-3-year-old children on disease burden within the general population: A Bayesian model-based approach
Abstract
Introduction: During the 2013-2014 influenza season, Public Health England extended routine influenza vaccination to all 2- and 3-year-old children in England. To estimate the impact of this change in policy on influenza-related morbidity and mortality, we developed a disease transmission and surveillance model informed by real-world data.
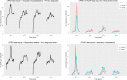
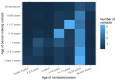
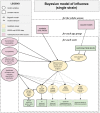
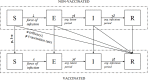
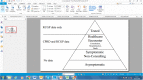
Methods: We combined real-world and literature data sources to construct a model of influenza transmission and surveillance in England. Data were obtained for four influenza seasons, starting with the 2010-2011 season. Bayesian inference was used to estimate model parameters on a season-by-season basis to assess the impact of targeting 2- and 3-year-old children for influenza vaccination. This provided the basis for the construction of counterfactual scenarios comparing vaccination rates of ~2% and ~35% in the 2- and 3- year-old population to estimate reductions in general practitioner (GP) influenza-like-illness (ILI) consultations, respiratory hospitalizations and deaths in the overall population.
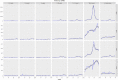
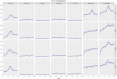
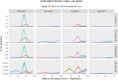
Results: Our model was able to replicate the main patterns of influenza across the four seasons as observed through laboratory surveillance data. Targeting 2- and 3-year-old children for influenza vaccination resulted in reductions in the general population of between 6.2-9.9% in influenza-attributable GP ILI consultations, 6.1-10.7% in influenza-attributable respiratory hospitalizations, and 5.7-9.4% in influenza-attributable deaths. The decrease in influenza-attributable ILI consultations represents a reduction of between 4.5% and 7.3% across all ILI consultations. The reduction in influenza-attributable respiratory hospitalizations represents a reduction of between 1.2% and 2.3% across all respiratory hospitalizations. Reductions in influenza-attributable respiratory deaths represent a reduction of between 0.9% and 2.4% in overall respiratory deaths.
Conclusion: This study has provided evidence that extending routine influenza vaccination to all healthy children aged 2 and 3 years old leads to benefits in terms of reduced utilization of healthcare resources and fewer respiratory health outcomes and deaths.
Conflict of interest statement
Figures


Similar articles
-
Assessing the burden of paediatric influenza in Europe: the European Paediatric Influenza Analysis (EPIA) project.Eur J Pediatr. 2010 Aug;169(8):997-1008. doi: 10.1007/s00431-010-1164-0. Epub 2010 Mar 13. Eur J Pediatr. 2010. PMID: 20229049 Free PMC article.
-
Influenza vaccine effectiveness estimates in Europe in a season with three influenza type/subtypes circulating: the I-MOVE multicentre case-control study, influenza season 2012/13.Euro Surveill. 2014 Feb 13;19(6):20701. doi: 10.2807/1560-7917.es2014.19.6.20701. Euro Surveill. 2014. PMID: 24556348
-
Effects of previous episodes of influenza and vaccination in preventing laboratory-confirmed influenza in Navarre, Spain, 2013/14 season.Euro Surveill. 2016 Jun 2;20(22). doi: 10.2807/1560-7917.ES.2016.21.22.30243. Euro Surveill. 2016. PMID: 27277013
-
Pediatric influenza and illness severity: what is known and what questions remain?Curr Opin Pediatr. 2019 Feb;31(1):119-126. doi: 10.1097/MOP.0000000000000721. Curr Opin Pediatr. 2019. PMID: 30531402 Review.
-
Impact of influenza vaccination on healthcare utilization - A systematic review.Vaccine. 2019 May 27;37(24):3179-3189. doi: 10.1016/j.vaccine.2019.04.051. Epub 2019 Apr 30. Vaccine. 2019. PMID: 31047677
Cited by
-
Cost-utility analysis of increasing uptake of universal seasonal quadrivalent influenza vaccine (QIV) in children aged 6 months and older in Germany.Hum Vaccin Immunother. 2022 Nov 30;18(5):2058304. doi: 10.1080/21645515.2022.2058304. Epub 2022 Apr 29. Hum Vaccin Immunother. 2022. PMID: 35486410 Free PMC article.
-
Vaccine hesitancy : Report of a student study group.Wien Klin Wochenschr. 2020 May;132(9-10):243-252. doi: 10.1007/s00508-020-01655-4. Epub 2020 Apr 22. Wien Klin Wochenschr. 2020. PMID: 32322962 Free PMC article. Review.
-
Seasonal influenza: Modelling approaches to capture immunity propagation.PLoS Comput Biol. 2019 Oct 28;15(10):e1007096. doi: 10.1371/journal.pcbi.1007096. eCollection 2019 Oct. PLoS Comput Biol. 2019. PMID: 31658250 Free PMC article.
References
-
- World Health Organization. Influenza (seasonal) 2014 [1 December 2016]. http://www.who.int/mediacentre/factsheets/fs211/en/.
-
- Hardelid P, Pebody R, Andrews N. Mortality caused by influenza and respiratory syncytial virus by age group in England and Wales 1999–2010. Influenza Other Respir Viruses. 2013;7(1):35–45. doi: 10.1111/j.1750-2659.2012.00345.x . - DOI - PMC - PubMed
-
- Cauchemez S, Valleron AJ, Boelle PY, Flahault A, Ferguson NM. Estimating the impact of school closure on influenza transmission from Sentinel data. Nature. 2008;452(7188):750–4. doi: 10.1038/nature06732 . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

