Constitutive Cyclin O deficiency results in penetrant hydrocephalus, impaired growth and infertility
- PMID: 29245899
- PMCID: PMC5725090
- DOI: 10.18632/oncotarget.21818
Constitutive Cyclin O deficiency results in penetrant hydrocephalus, impaired growth and infertility
Abstract
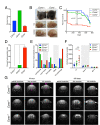
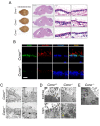
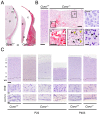
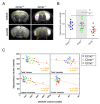
Cyclin O (encoded by CCNO) is a member of the cyclin family with regulatory functions in ciliogenesis and apoptosis. Homozygous CCNO mutations have been identified in human patients with Reduced Generation of Multiple Motile Cilia (RGMC) and conditional inactivation of Ccno in the mouse recapitulates some of the pathologies associated with the human disease. These include defects in the development of motile cilia and hydrocephalus. To further investigate the functions of Ccno in vivo, we have generated a new mouse model characterized by the constitutive loss of Ccno in all tissues and followed a cohort during ageing. Ccno-/- mice were growth impaired and developed hydrocephalus with high penetrance. In addition, some Ccno+/- mice also developed hydrocephalus and affected Ccno-/- and Ccno+/- mice exhibited additional CNS defects including cortical thinning and hippocampal abnormalities. In addition to the CNS defects, both male and female Ccno-/- mice were infertile and female mice exhibited few motile cilia in the oviduct. Our results further establish CCNO as an important gene for normal development and suggest that heterozygous CCNO mutations could underlie hydrocephalus or diminished fertility in some human patients.
Keywords: Cyclin O; Gerotarget; ciliogenesis; development; hydrocephalus; neurogenesis.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that they have no conflict of interest.
Figures
References
-
- Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140:3079–3093. - PubMed
-
- Granes F, Roig MB, Brady HJ, Gil-Gomez G. Cdk2 activation acts upstream of the mitochondrion during glucocorticoid induced thymocyte apoptosis. Eur J Immunol. 2004;34:2781–2790. - PubMed
-
- Roig MB, Roset R, Ortet L, Balsiger NA, Anfosso A, Cabellos L, Garrido M, Alameda F, Brady HJ, Gil-Gomez G. Identification of a novel cyclin required for the intrinsic apoptosis pathway in lymphoid cells. Cell Death Differ. 2009;16:230–243. - PubMed
-
- Wallmeier J, Al-Mutairi DA, Chen CT, Loges NT, Pennekamp P, Menchen T, Ma L, Shamseldin HE, Olbrich H, Dougherty GW, Werner C, Alsabah BH, Kohler G, et al. Mutations in CCNO result in congenital mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat Genet. 2014;46:646–651. - PubMed
-
- Amirav I, Wallmeier J, Loges NT, Menchen T, Pennekamp P, Mussaffi H, Abitbul R, Avital A, Bentur L, Dougherty GW, Nael E, Lavie M, Olbrich H, et al. Systematic Analysis of CCNO Variants in a Defined Population: Implications for Clinical Phenotype and Differential Diagnosis. Hum Mutat. 2016;37:396–405. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

