Venom-based peptide therapy: insights into anti-cancer mechanism
- PMID: 29246030
- PMCID: PMC5725072
- DOI: 10.18632/oncotarget.21740
Venom-based peptide therapy: insights into anti-cancer mechanism
Abstract
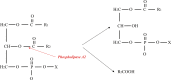
The 5-year relative survival rate of all types of cancer has increased significantly over the past three decades partly due to the targeted therapy. However, still there are many targeted therapy drugs could play a role only in a portion of cancer patients with specific molecular alternation. It is necessary to continue to develop new biological agents which could be used alone and/or in combination with current FDA approved drugs to treat complex cancer diseases. Venom-based drugs have been used for hundreds of years in human history. Nevertheless, the venom-origin of the anti-cancer drug do rarely appear in the pharmaceutical market; and this is due to the fact that the mechanism of action for a large number of the venom drug such as venom-based peptide is not clearly understood. In this review, we focus on discussing some identified venom-based peptides and their anti-cancer mechanisms including the blockade of cancer cell proliferation, invasion, angiogenesis, and metastasis (hallmarks of cancer) to fulfill the gap which is hindering their use in cancer therapy. Furthermore, it also highlights the importance of immunotherapy based on venom peptide. Overall, this review provides readers for further understanding the mechanism of venom peptide and elaborates on the need to explore peptide-based therapeutic strategies.
Keywords: anticancer mechanism; metastasis; signaling pathway; targeted therapy; venom.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures


References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. https://doi.org/10.3322/caac.21262. - DOI - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. https://doi.org/10.3322/caac.21387. - DOI - PubMed
-
- Hashim D, Boffetta P, La Vecchia C, Rota M, Bertuccio P, Malvezzi M, Negri E. The global decrease in cancer mortality: trends and disparities. Ann Oncol. 2016;27:926–33. https://doi.org/10.1093/annonc/mdw027. - DOI - PubMed
-
- King GF. Venoms as a platform for human drugs: translating toxins into therapeutics. Expert Opin Biol Ther. 2011;11:1469–84. https://doi.org/10.1517/14712598.2011.621940. - DOI - PubMed
-
- Newman DJ, Cragg GM. Natural Products as Sources of New Drugs from 1981 to 2014. J Nat Prod. 2016;79:629–61. https://doi.org/10.1021/acs.jnatprod.5b01055. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

