Dexamethasone implants in paediatric patients with noninfectious intermediate or posterior uveitis: first prospective exploratory case series
- PMID: 29246154
- PMCID: PMC5732406
- DOI: 10.1186/s12886-017-0648-3
Dexamethasone implants in paediatric patients with noninfectious intermediate or posterior uveitis: first prospective exploratory case series
Abstract
Background: To evaluate the efficacy and safety of dexamethasone (DEX) implants in paediatric patients with noninfectious intermediate or posterior uveitis.
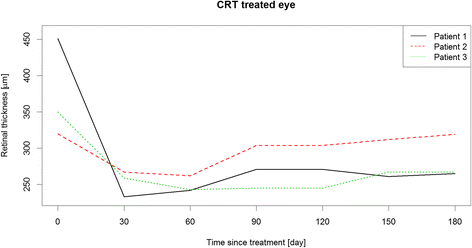
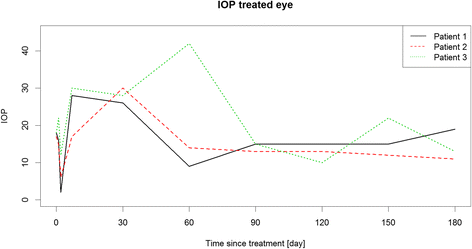
Methods: Prospective single center exploratory case series. Children and adolescents, 6 to 17 years old, with a vitreous haze score of ≥1.5+ or cystoid macular edema (CME) of >300 μm were enrolled. Vitreous haze score at month 2 was chosen as primary endpoint. Best corrected visual acuity (BCVA), central retinal thickness (CRT) and concomitant medication at month 6 were defined as secondary endpoints. Intraocular pressure (IOP) and cataract formation were determined as safety endpoints.
Results: Three out of 6 eligible patients participated in the case series. At month 2, vitreous haze was reduced from a score of 1.5+ to 0.5+ and 0 and BCVA improved by ≥3 lines, ≥4 lines and ≥2 lines of Early Treatment of Diabetic Retinopathy (ETDRS)-letters, respectively. Visual acuity gain was accompanied by a CRT reduction of -186 μm and -83 μm in the first and third patient, in whom CME was the indication for DEX implantation. A reduction of concomitant medication was achieved in 1 patient. IOP increase was seen in all 3 patients, but could be treated sufficiently with primarily IOP lowering medications and without need for glaucoma surgery. Cataract progression did not occur.
Conclusions: DEX implants led to an improvement in all endpoints, especially BCVA. This study confirms that IOP rises may also occur in the paediatric population and should be monitored and treated appropriately.
Trial registration: European Union Drug Regulating Authorities Clinical Trials (EudraCT)- nr: 2013-000541-39.
Keywords: Cataract; Dexamethasone implant; Glaucoma; Macular edema; Paediatric patients; Uveitis; Vitreous haze.
Conflict of interest statement
Ethics approval and consent to participate
Approval of the German Institute for Medicinal Products and Medical Devices (BfArM) and the Institutional Review Board (IRB) of the Charité-Universitätsmedizin Berlin were obtained (13/0282 – EK 15). The case series was registered under the European Union Drug Regulating Autorities Clinical Trials (EudraCT) number 2013–000541-39 and was conducted in concordance with the good clinical practice (GCP) regulations and the German Law for Medicinal Products (AMG).
Consent for publication
Written informed consent was obtained from the patients or their parents for publication of this case series. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Heiligenhaus A, Niewerth M, Mingels A, et al. Epidemiology of uveitis in juvenile idiopathic arthritis from a national paediatric rheumatologic and opthalmologic database. KlinMonblAugenheilkd. 2005;222(12):993–1001. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials