Airway administration of corticosteroids for prevention of bronchopulmonary dysplasia in premature infants: a meta-analysis with trial sequential analysis
- PMID: 29246209
- PMCID: PMC5732371
- DOI: 10.1186/s12890-017-0550-z
Airway administration of corticosteroids for prevention of bronchopulmonary dysplasia in premature infants: a meta-analysis with trial sequential analysis
Abstract
Background: Uncertainly prevails with regard to the use of inhalation or instillation steroids to prevent bronchopulmonary dysplasia in preterm infants. The meta-analysis with sequential analysis was designed to evaluate the efficacy and safety of airway administration (inhalation or instillation) of corticosteroids for preventing bronchopulmonary dysplasia (BPD) in premature infants.
Methods: We searched MEDLINE, EMBASE, CINAHL, and Cochrane CENTRAL from their inceptions to February 2017. All published randomized controlled trials (RCTs) evaluating the effect of airway administration of corticosteroids (AACs) vs placebo or systemic corticosteroid in prematurity were included. All meta-analyses were performed using Review Manager 5.3.
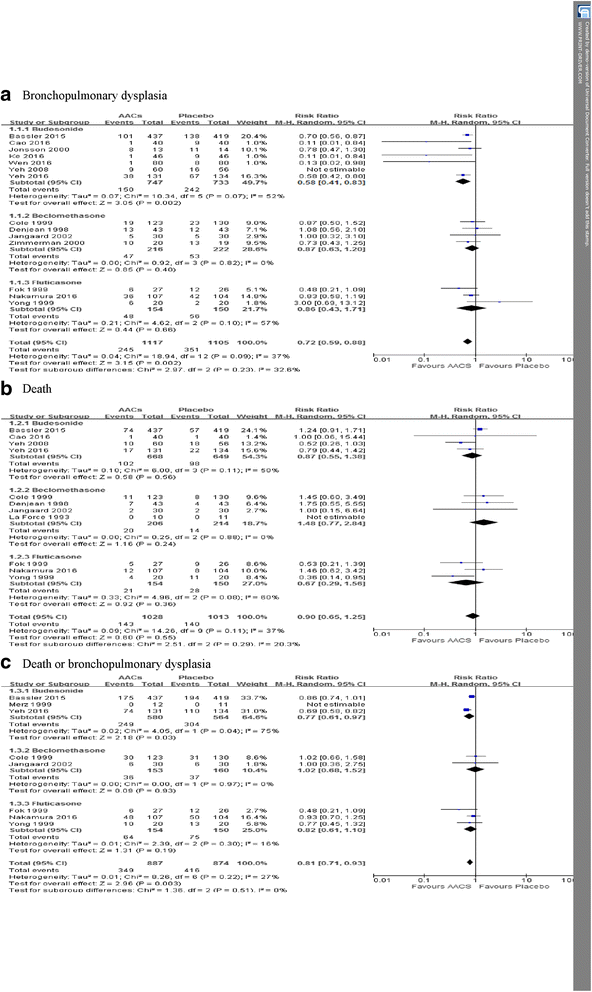
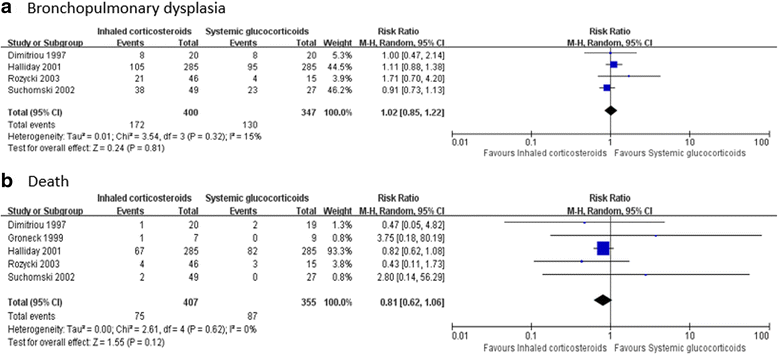
Results: Twenty five RCTs retrieved (n = 3249) were eligible for further analysis. Meta-analysis and trial sequential analysis corrected the 95% confidence intervals estimated a lower risk of the primary outcome of BPD (relative risk 0.71, adjusted 95% confidence interval 0.57-0.87) and death or BPD (relative risk 0.81, adjusted 95% confidence interval 0.71-0.97) in AACs group than placebo and it is equivalent for preventing BPD than systemic corticosteroids. Moreover, AACs fail to increasing risk of death compared with placebo (relative risk 0.90, adjusted 95% confidence interval 0.40-2.03) or systemic corticosteroids (relative risk 0.81, 95% confidence interval 0.62-1.06).
Conclusions: Our findings suggests that AACs (especially instillation of budesonide using surfactant as a vehicle) are an effective and safe option for preventing BPD in preterm infants. Furthermore, the appropriate dose and duration, inhalation or instillation with surfactant as a vehicle and the long-term safety of airway administration of corticosteroids needs to be assessed in large trials.
Keywords: Bronchopulmonary dysplasia; Inhaled corticosteroids; Meta-analysis; Neurodevelopmental outcomes; Preterm infants.
Conflict of interest statement
Ethics approval and consent to participate
As the paper did not involve any human or animal, the ethical approval was not required.
Consent for publication
Not applicable.
Competing interests
None of the investigators declare any real or perceived conflicts of interest pertaining to the subject of this manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





Similar articles
-
Early Intratracheal Administration of Corticosteroid and Pulmonary Surfactant for Preventing Bronchopulmonary Dysplasia in Preterm Infants with Neonatal Respiratory Distress Syndrome: A Meta-analysis.Curr Med Sci. 2019 Jun;39(3):493-499. doi: 10.1007/s11596-019-2064-9. Epub 2019 Jun 17. Curr Med Sci. 2019. PMID: 31209823
-
Efficacy and safety of different inhaled corticosteroids for bronchopulmonary dysplasia prevention in preterm infants: A systematic review and meta-analysis.Respir Med Res. 2024 Jun;85:101096. doi: 10.1016/j.resmer.2024.101096. Epub 2024 Feb 29. Respir Med Res. 2024. PMID: 38744231
-
Inhalation or instillation of steroids for the prevention of bronchopulmonary dysplasia.Neonatology. 2015;107(4):358-9. doi: 10.1159/000381132. Epub 2015 Jun 5. Neonatology. 2015. PMID: 26044104 Review.
-
Intratracheal budesonide mixed with surfactant to increase survival free of bronchopulmonary dysplasia in extremely preterm infants: study protocol for the international, multicenter, randomized PLUSS trial.Trials. 2023 May 9;24(1):320. doi: 10.1186/s13063-023-07257-5. Trials. 2023. PMID: 37161488 Free PMC article.
-
Long-term effects of the intratracheal administration of corticosteroids for the prevention of bronchopulmonary dysplasia: A meta-analysis.Pediatr Pulmonol. 2019 Nov;54(11):1722-1734. doi: 10.1002/ppul.24452. Epub 2019 Aug 9. Pediatr Pulmonol. 2019. PMID: 31397120
Cited by
-
Early Intratracheal Administration of Corticosteroid and Pulmonary Surfactant for Preventing Bronchopulmonary Dysplasia in Preterm Infants with Neonatal Respiratory Distress Syndrome: A Meta-analysis.Curr Med Sci. 2019 Jun;39(3):493-499. doi: 10.1007/s11596-019-2064-9. Epub 2019 Jun 17. Curr Med Sci. 2019. PMID: 31209823
-
A Randomized Clinical Trial of Intratracheal Administration of Surfactant and Budesonide Combination in Comparison to Surfactant for Prevention of Bronchopulmonary Dysplasia.Oman Med J. 2021 Jul 31;36(4):e289. doi: 10.5001/omj.2021.84. eCollection 2021 Jul. Oman Med J. 2021. PMID: 34447583 Free PMC article.
-
Corticosteroids for the prevention and treatment of bronchopulmonary dysplasia: an overview of systematic reviews.Cochrane Database Syst Rev. 2024 Apr 10;4(4):CD013271. doi: 10.1002/14651858.CD013271.pub2. Cochrane Database Syst Rev. 2024. PMID: 38597338 Free PMC article. Review.
-
Comparison of decision-making in neonatal care between China and Japan.World J Pediatr. 2019 Feb;15(1):85-91. doi: 10.1007/s12519-018-0211-1. Epub 2018 Nov 23. World J Pediatr. 2019. PMID: 30470979
-
Comparison of two novel diagnostic criteria for bronchopulmonary dysplasia in predicting adverse outcomes of preterm infants: a retrospective cohort study.BMC Pulm Med. 2023 Aug 23;23(1):308. doi: 10.1186/s12890-023-02590-6. BMC Pulm Med. 2023. PMID: 37612680 Free PMC article.
References
-
- Short EJ, Kirchner HL, Asaad GR, Fulton SE, Lewis BA, Klein N, et al. Developmental sequelae in preterm infants having a diagnosis of bronchopulmonary dysplasia: analysis using a severity-based classification system. Arch Pediatr Adolesc Med. 2007;161:1082–1087. doi: 10.1001/archpedi.161.11.1082. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

