Oligomeric forms of amyloid-β protein in plasma as a potential blood-based biomarker for Alzheimer's disease
- PMID: 29246249
- PMCID: PMC5732503
- DOI: 10.1186/s13195-017-0324-0
Oligomeric forms of amyloid-β protein in plasma as a potential blood-based biomarker for Alzheimer's disease
Abstract
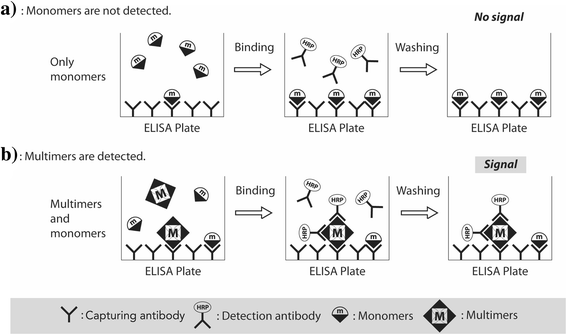
Background: Soluble amyloid-β (Aβ) oligomers are the major toxic substances associated with the pathology of Alzheimer's disease (AD). The ability to measure Aβ oligomer levels in the blood would provide simple and minimally invasive tools for AD diagnostics. In the present study, the recently developed Multimer Detection System (MDS) for AD, a new enzyme-linked immunosorbent assay for measuring Aβ oligomers selectively, was used to detect Aβ oligomers in the plasma of patients with AD and healthy control individuals.
Methods: Twenty-four patients with AD and 37 cognitively normal control individuals underwent extensive clinical evaluations as follows: blood sampling; detailed neuropsychological tests; brain magnetic resonance imaging; cerebrospinal fluid (CSF) measurement of Aβ42, phosphorylated tau protein (pTau), and total tau protein (tTau); and 11C-Pittsburgh compound B (PIB) positron emission tomography. Pearson's correlation analyses between the estimations of Aβ oligomer levels by MDS and other conventional AD biomarkers (CSF Aβ42, pTau, and tTau, as well as PIB standardized uptake value ratio [PIB SUVR]) were conducted. ROC analyses were used to compare the diagnostic performance of each biomarker.
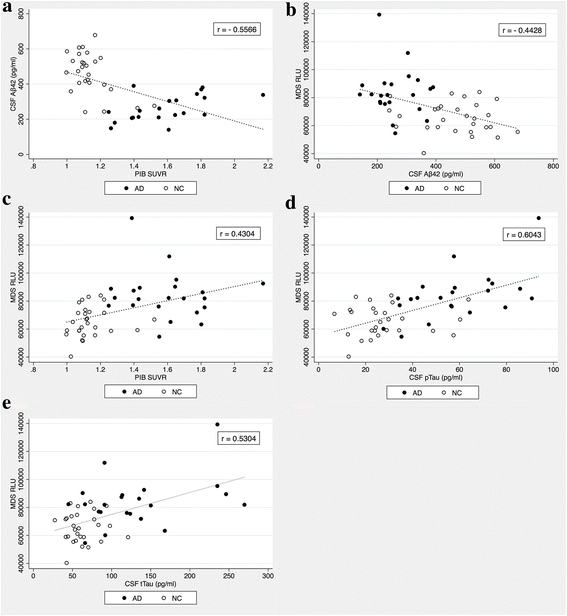
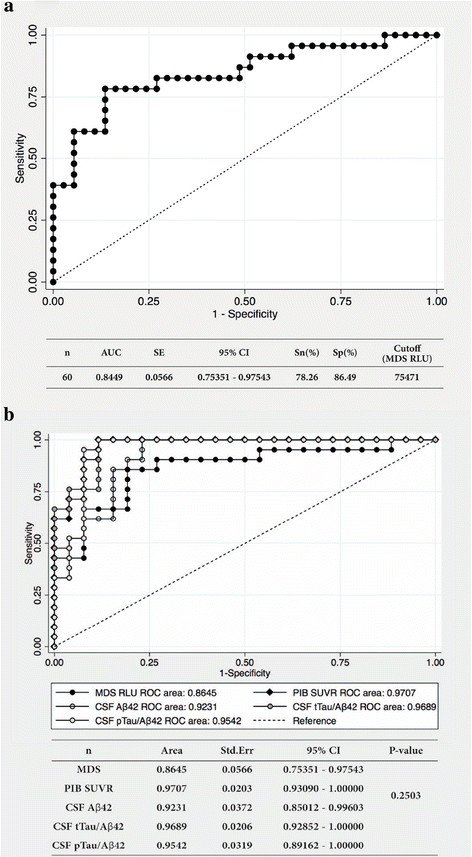
Results: The plasma levels of Aβ oligomers by MDS were higher in patients with AD than in normal control individuals, and they correlated well with conventional AD biomarkers (levels of Aβ oligomers by MDS vs. CSF Aβ42, r = -0.443; PIB SUVR, r = 0.430; CSF pTau, r = 0.530; CSF tTau, r = 0.604). The sensitivity and specificity of detecting plasma Aβ oligomers by MDS for differentiating AD from the normal controls were 78.3% and 86.5%, respectively. The AUC for plasma Aβ oligomers by MDS was 0.844, which was not significantly different from the AUC of other biomarkers (p = 0.250).
Conclusions: Plasma levels of Aβ oligomers could be assessed using MDS, which might be a simple, noninvasive, and accessible assay for evaluating brain amyloid deposition related to AD pathology.
Keywords: Alzheimer’s disease; Amyloid-β protein; Biomarker; Oligomer.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the institutional review board of the Seoul National University Bundang Hospital and Chung-Ang University Hospital [B-1202-145-003, B-0905-075-003, C2013142(1102), C2012048(743)]. Written informed consent was obtained from all patients, or their caregivers, who participated in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Alzheimer's Disease Normative Cerebrospinal Fluid Biomarkers Validated in PET Amyloid-β Characterized Subjects from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study.J Alzheimers Dis. 2015;48(1):175-87. doi: 10.3233/JAD-150247. J Alzheimers Dis. 2015. PMID: 26401938
-
Plasma Aβ but not tau is related to brain PiB retention in early Alzheimer's disease.ACS Chem Neurosci. 2014 Sep 17;5(9):830-6. doi: 10.1021/cn500101j. Epub 2014 Aug 13. ACS Chem Neurosci. 2014. PMID: 25054847
-
Concordance between cerebrospinal fluid biomarkers and [11C]PIB PET in a memory clinic cohort.J Alzheimers Dis. 2014;41(3):801-7. doi: 10.3233/JAD-132561. J Alzheimers Dis. 2014. PMID: 24705549
-
Development of molecular tools for diagnosis of Alzheimer's disease that are based on detection of amyloidogenic proteins.Prion. 2021 Dec;15(1):56-69. doi: 10.1080/19336896.2021.1917289. Prion. 2021. PMID: 33910450 Free PMC article. Review.
-
Simultaneous analysis of cerebrospinal fluid biomarkers using microsphere-based xMAP multiplex technology for early detection of Alzheimer's disease.Methods. 2012 Apr;56(4):484-93. doi: 10.1016/j.ymeth.2012.03.023. Epub 2012 Apr 6. Methods. 2012. PMID: 22503777 Review.
Cited by
-
Plasma lipidome is dysregulated in Alzheimer's disease and is associated with disease risk genes.Transl Psychiatry. 2021 Jun 7;11(1):344. doi: 10.1038/s41398-021-01362-2. Transl Psychiatry. 2021. PMID: 34092785 Free PMC article.
-
Human Palatine Tonsils Are Linked to Alzheimer's Disease through Function of Reservoir of Amyloid Beta Protein Associated with Bacterial Infection.Cells. 2022 Jul 24;11(15):2285. doi: 10.3390/cells11152285. Cells. 2022. PMID: 35892582 Free PMC article.
-
Dysregulated expression levels of APH1B in peripheral blood are associated with brain atrophy and amyloid-β deposition in Alzheimer's disease.Alzheimers Res Ther. 2021 Nov 3;13(1):183. doi: 10.1186/s13195-021-00919-z. Alzheimers Res Ther. 2021. PMID: 34732252 Free PMC article.
-
Blood amyloid-β oligomerization associated with neurodegeneration of Alzheimer's disease.Alzheimers Res Ther. 2019 May 10;11(1):40. doi: 10.1186/s13195-019-0499-7. Alzheimers Res Ther. 2019. PMID: 31077246 Free PMC article.
-
Reference interval and the role of plasma oligomeric beta amyloid in screening of risk groups for cognitive dysfunction at health checkups.J Clin Lab Anal. 2021 Sep;35(9):e23933. doi: 10.1002/jcla.23933. Epub 2021 Aug 3. J Clin Lab Anal. 2021. PMID: 34342379 Free PMC article.
References
-
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. - DOI - PMC - PubMed
-
- Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, Krafft GA, Klein WL. Alzheimer’s disease-affected brain: presence of oligomeric Aβ ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci U S A. 2003;100:10417–22. doi: 10.1073/pnas.1834302100. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

