Cystic Fibrosis Transmembrane Conductance Regulator Attaches Tumor Suppressor PTEN to the Membrane and Promotes Anti Pseudomonas aeruginosa Immunity
- PMID: 29246444
- PMCID: PMC5738266
- DOI: 10.1016/j.immuni.2017.11.010
Cystic Fibrosis Transmembrane Conductance Regulator Attaches Tumor Suppressor PTEN to the Membrane and Promotes Anti Pseudomonas aeruginosa Immunity
Abstract
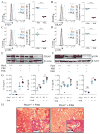
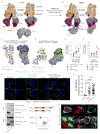
The tumor suppressor PTEN controls cell proliferation by regulating phosphatidylinositol-3-kinase (PI3K) activity, but the participation of PTEN in host defense against bacterial infection is less well understood. Anti-inflammatory PI3K-Akt signaling is suppressed in patients with cystic fibrosis (CF), a disease characterized by hyper-inflammatory responses to airway infection. We found that Ptenl-/- mice, which lack the NH2-amino terminal splice variant of PTEN, were unable to eradicate Pseudomonas aeruginosa from the airways and could not generate sufficient anti-inflammatory PI3K activity, similar to what is observed in CF. PTEN and the CF transmembrane conductance regulator (CFTR) interacted directly and this interaction was necessary to position PTEN at the membrane. CF patients under corrector-potentiator therapy, which enhances CFTR transport to the membrane, have increased PTEN amounts. These findings suggest that improved CFTR trafficking could enhance P. aeruginosa clearance from the CF airway by activating PTEN-mediated anti-bacterial responses and might represent a therapeutic strategy.
Keywords: CFTR; NF-κB; PI3K; PTEN; Pseudomonas aeruginosa.
Published by Elsevier Inc.
Conflict of interest statement
Figures




Comment in
-
PTENtiating CFTR for Antimicrobial Immunity.Immunity. 2017 Dec 19;47(6):1014-1016. doi: 10.1016/j.immuni.2017.12.002. Immunity. 2017. PMID: 29262343
References
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. - PubMed
-
- Aksoy E, Taboubi S, Torres D, Delbauve S, Hachani A, Whitehead MA, Pearce WP, Berenjeno IM, Nock G, Filloux A, et al. The p110delta isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock. Nat Immunol. 2012;13:1045–1054. - PMC - PubMed
-
- Bae YS, Lee HY, Jung YS, Lee M, Suh PG. Phospholipase Cgamma in Toll-like receptor-mediated inflammation and innate immunity. Adv Biol Regul. 2017;63:92–97. - PubMed
-
- Boyle MP, Bell SC, Konstan MW, McColley SA, Rowe SM, Rietschel E, Huang X, Waltz D, Patel NR, Rodman D, et al. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: a phase 2 randomised controlled trial. Lancet Respir Med. 2014;2:527–538. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

